Translation of Polymeric Microneedles for Treatment of Human Diseases: Recent Trends, Progress, and Challenges
- PMID: 34452093
- PMCID: PMC8401662
- DOI: 10.3390/pharmaceutics13081132
Translation of Polymeric Microneedles for Treatment of Human Diseases: Recent Trends, Progress, and Challenges
Abstract
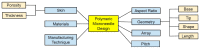
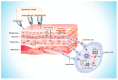
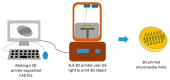
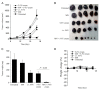
The ongoing search for biodegradable and biocompatible microneedles (MNs) that are strong enough to penetrate skin barriers, easy to prepare, and can be translated for clinical use continues. As such, this review paper is focused upon discussing the key points (e.g., choice polymeric MNs) for the translation of MNs from laboratory to clinical practice. The review reveals that polymers are most appropriately used for dissolvable and swellable MNs due to their wide range of tunable properties and that natural polymers are an ideal material choice as they structurally mimic native cellular environments. It has also been concluded that natural and synthetic polymer combinations are useful as polymers usually lack mechanical strength, stability, or other desired properties for the fabrication and insertion of MNs. This review evaluates fabrication methods and materials choice, disease and health conditions, clinical challenges, and the future of MNs in public healthcare services, focusing on literature from the last decade.
Keywords: human disease; polymeric microneedle; transdermal drug delivery; vaccine delivery.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

