Aberrant Neurogliovascular Unit Dynamics in Cerebral Small Vessel Disease: A Rheological Clue to Vascular Parkinsonism
- PMID: 34452169
- PMCID: PMC8398765
- DOI: 10.3390/pharmaceutics13081207
Aberrant Neurogliovascular Unit Dynamics in Cerebral Small Vessel Disease: A Rheological Clue to Vascular Parkinsonism
Abstract
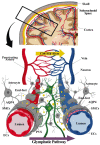
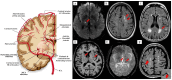
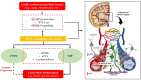
The distinctive anatomical assemble and functionally discrete multicellular cerebrovasculature dynamics confer varying rheological and blood-brain barrier permeabilities to preserve the integrity of cerebral white matter and its neural microenvironment. This homeostasis intricately involves the glymphatic system that manages the flow of interstitial solutes, metabolic waste, and clearance through the venous circulation. As a physiologically integrated neurogliovascular unit (NGVU) serving a particularly vulnerable cerebral white matter (from hypoxia, metabolic insults, infection, and inflammation), a likely insidious process over a lifetime could inflict microenvironment damages that may lead to pathological conditions. Two such conditions, cerebral small vessel disease (CSVD) and vascular parkinsonism (VaP), with poorly understood pathomechanisms, are frequently linked to this brain-wide NGVU. VaP is widely regarded as an atypical parkinsonism, described by cardinal motor manifestations and the presence of cerebrovascular disease, particularly white matter hyperintensities (WMHs) in the basal ganglia and subcortical region. WMHs, in turn, are a recognised imaging spectrum of CSVD manifestations, and in relation to disrupted NGVU, also include enlarged perivascular spaces. Here, in this narrative review, we present and discuss on recent findings that argue for plausible clues between CSVD and VaP by focusing on aberrant multicellular dynamics of a unique integrated NGVU-a crossroad of the immune-vascular-nervous system-which may also extend fresher insights into the elusive interplay between cerebral microvasculature and neurodegeneration, and the potential therapeutic targets.
Keywords: cerebral small vessel disease; glymphatic system; homeostasis; neurogliovascular unit; vascular parkinsonism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Is cerebral small vessel disease a central nervous system interstitial fluidopathy?IBRO Neurosci Rep. 2023 Dec 23;16:98-105. doi: 10.1016/j.ibneur.2023.12.004. eCollection 2024 Jun. IBRO Neurosci Rep. 2023. PMID: 39007087 Free PMC article.
-
Neurovascular-glymphatic dysfunction and white matter lesions.Geroscience. 2021 Aug;43(4):1635-1642. doi: 10.1007/s11357-021-00361-x. Epub 2021 Apr 14. Geroscience. 2021. PMID: 33851307 Free PMC article.
-
Parkinsonism and cerebrovascular disease.J Neurol Sci. 2022 Feb 15;433:120011. doi: 10.1016/j.jns.2021.120011. Epub 2021 Oct 6. J Neurol Sci. 2022. PMID: 34686356 Review.
-
Correlations of Plasma Biomarkers and Imaging Characteristics of Cerebral Small Vessel Disease.Brain Sci. 2024 Mar 12;14(3):269. doi: 10.3390/brainsci14030269. Brain Sci. 2024. PMID: 38539657 Free PMC article. Review.
-
Advances in the Role of Endothelial Cells in Cerebral Small Vessel Disease.Front Neurol. 2022 Apr 11;13:861714. doi: 10.3389/fneur.2022.861714. eCollection 2022. Front Neurol. 2022. PMID: 35481273 Free PMC article. Review.
Cited by
-
Association of enlarged perivascular spaces with upper extremities and gait impairment: An observational, prospective cohort study.Front Neurol. 2022 Oct 26;13:993979. doi: 10.3389/fneur.2022.993979. eCollection 2022. Front Neurol. 2022. PMID: 36388205 Free PMC article.
-
The Characteristics of the Metabolomic Profile in Patients with Parkinson's Disease and Vascular Parkinsonism.Acta Naturae. 2024 Oct-Dec;16(4):27-37. doi: 10.32607/actanaturae.27511. Acta Naturae. 2024. PMID: 39877011 Free PMC article.
-
Association Between Large Numbers of Enlarged Perivascular Spaces in Basal Ganglia and Motor Performance in Elderly Individuals: A Cross-Sectional Study.Clin Interv Aging. 2022 Jun 2;17:903-913. doi: 10.2147/CIA.S364794. eCollection 2022. Clin Interv Aging. 2022. PMID: 35677185 Free PMC article.
-
The Microbiota-Gut-Brain Axis: Key Mechanisms Driving Glymphopathy and Cerebral Small Vessel Disease.Life (Basel). 2024 Dec 24;15(1):3. doi: 10.3390/life15010003. Life (Basel). 2024. PMID: 39859943 Free PMC article. Review.
-
Cerebral small vessel disease: The impact of glymphopathy and sleep disorders.J Cereb Blood Flow Metab. 2025 May 5:271678X251333933. doi: 10.1177/0271678X251333933. Online ahead of print. J Cereb Blood Flow Metab. 2025. PMID: 40322968 Free PMC article. Review.
References
-
- Louveau A., Herz J., Alme M.N., Salvador A.F., Dong M.Q., Viar K.E., Herod S.G., Knopp J., Setliff J.C., Lupi A.L., et al. CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat. Neurosci. 2018;21:1380–1391. doi: 10.1038/s41593-018-0227-9. - DOI - PMC - PubMed
-
- Lammie A.G. Small vessel disease. In: Kalimo H., editor. Cerebrovascular Diseases. ISN Neuropath Press; Basel, Switzerland: 2005. pp. 85–91.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

