Magnetoliposomes Based on Shape Anisotropic Calcium/Magnesium Ferrite Nanoparticles as Nanocarriers for Doxorubicin
- PMID: 34452210
- PMCID: PMC8401618
- DOI: 10.3390/pharmaceutics13081248
Magnetoliposomes Based on Shape Anisotropic Calcium/Magnesium Ferrite Nanoparticles as Nanocarriers for Doxorubicin
Abstract
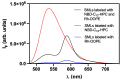
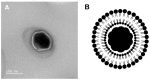
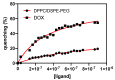
Multifunctional lipid nanocarriers are a promising therapeutic approach for controlled drug release in cancer therapy. Combining the widely used liposome structure with magnetic nanoparticles in magnetoliposomes allies, the advantages of using liposomes include the possibility to magnetically guide, selectively accumulate, and magnetically control the release of drugs on target. The effectiveness of these nanosystems is intrinsically related to the individual characteristics of the two main components-lipid formulation and magnetic nanoparticles-and their physicochemical combination. Herein, shape-anisotropic calcium-substituted magnesium ferrite nanoparticles (Ca0.25Mg0.75Fe2O4) were prepared for the first time, improving the magnetic properties of spherical counterparts. The nanoparticles revealed a superparamagnetic behavior, high saturation magnetization (50.07 emu/g at 300 K), and a large heating capacity. Furthermore, a new method for the synthesis of solid magnetoliposomes (SMLs) was developed to enhance their magnetic response. The manufacturing technicalities were optimized with different lipid compositions (DPPC, DPPC/Ch, and DPPC/DSPE-PEG) originating nanosystems with optimal sizes for biomedical applications (around or below 150 nm) and low polydispersity index. The high encapsulation efficiency of doxorubicin in these magnetoliposomes was proven, as well as the ability of the drug-loaded nanosystems to interact with cell membrane models and release DOX by fusion. SMLs revealed to reduce doxorubicin interaction with human serum albumin, contributing to a prolonged bioavailability of the drug upon systemic administration. Finally, the drug release kinetic assays revealed a preferable DOX release at hyperthermia temperatures (42 °C) and acidic conditions (pH = 5.5), indicating them as promising controlled release nanocarriers by either internal (pH) and external (alternate magnetic field) stimuli in cancer therapy.
Keywords: doxorubicin; magnetic hyperthermia; magnetic nanoparticles; magnetoliposomes; mixed ferrites; shape-anisotropy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures














Similar articles
-
Magnetoliposomes with Calcium-Doped Magnesium Ferrites Anchored in the Lipid Surface for Enhanced DOX Release.Nanomaterials (Basel). 2023 Sep 20;13(18):2597. doi: 10.3390/nano13182597. Nanomaterials (Basel). 2023. PMID: 37764626 Free PMC article.
-
Development of pH-Sensitive Magnetoliposomes Containing Shape Anisotropic Nanoparticles for Potential Application in Combined Cancer Therapy.Nanomaterials (Basel). 2023 Mar 15;13(6):1051. doi: 10.3390/nano13061051. Nanomaterials (Basel). 2023. PMID: 36985945 Free PMC article.
-
Stealth Magnetoliposomes Based on Calcium-Substituted Magnesium Ferrite Nanoparticles for Curcumin Transport and Release.Int J Mol Sci. 2020 May 21;21(10):3641. doi: 10.3390/ijms21103641. Int J Mol Sci. 2020. PMID: 32455630 Free PMC article.
-
Magnetoliposomes: recent advances in the field of controlled drug delivery.Expert Opin Drug Deliv. 2021 Oct;18(10):1323-1334. doi: 10.1080/17425247.2021.1915983. Epub 2021 Apr 21. Expert Opin Drug Deliv. 2021. PMID: 33836636 Review.
-
Magnetoliposomes in Controlled-Release Drug Delivery Systems.Crit Rev Biomed Eng. 2019;47(6):495-505. doi: 10.1615/CritRevBiomedEng.2020033002. Crit Rev Biomed Eng. 2019. PMID: 32421974 Free PMC article. Review.
Cited by
-
Targeting nanoplatform synergistic glutathione depletion-enhanced chemodynamic, microwave dynamic, and selective-microwave thermal to treat lung cancer bone metastasis.Bioact Mater. 2024 May 30;39:544-561. doi: 10.1016/j.bioactmat.2024.04.016. eCollection 2024 Sep. Bioact Mater. 2024. PMID: 38883314 Free PMC article.
-
Electrospun Magnetic Nanofiber Mats for Magnetic Hyperthermia in Cancer Treatment Applications-Technology, Mechanism, and Materials.Polymers (Basel). 2023 Apr 15;15(8):1902. doi: 10.3390/polym15081902. Polymers (Basel). 2023. PMID: 37112049 Free PMC article. Review.
-
Solid Magnetoliposomes as Multi-Stimuli-Responsive Systems for Controlled Release of Doxorubicin: Assessment of Lipid Formulations.Biomedicines. 2022 May 23;10(5):1207. doi: 10.3390/biomedicines10051207. Biomedicines. 2022. PMID: 35625942 Free PMC article.
-
A Compendium of Magnetic Nanoparticle Essentials: A Comprehensive Guide for Beginners and Experts.Pharmaceutics. 2025 Jan 20;17(1):137. doi: 10.3390/pharmaceutics17010137. Pharmaceutics. 2025. PMID: 39861783 Free PMC article. Review.
-
The Therapeutic Potential of Chemo/Thermotherapy with Magnetoliposomes for Cancer Treatment.Pharmaceutics. 2022 Nov 11;14(11):2443. doi: 10.3390/pharmaceutics14112443. Pharmaceutics. 2022. PMID: 36432634 Free PMC article. Review.
References
Grants and funding
- UIDB/04650/2020/Fundação para a Ciência e a Tecnologia
- PTDC/QUI-QFI/28020/2017/Fundação para a Ciência e a Tecnologia
- SFRH/BD/141936/2018/Fundação para a Ciência e a Tecnologia
- POCI-01-0145-FEDER-028020/European Regional Development Fund
- POCI-01-0145-FEDER-028020/Programa Operacional Temático Factores de Competitividade
LinkOut - more resources
Full Text Sources

