Bioinspired Magnetic Nanochains for Medicine
- PMID: 34452223
- PMCID: PMC8398308
- DOI: 10.3390/pharmaceutics13081262
Bioinspired Magnetic Nanochains for Medicine
Abstract
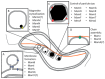
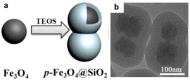
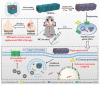
Superparamagnetic iron oxide nanoparticles (SPIONs) have been widely used for medicine, both in therapy and diagnosis. Their guided assembly into anisotropic structures, such as nanochains, has recently opened new research avenues; for instance, targeted drug delivery. Interestingly, magnetic nanochains do occur in nature, and they are thought to be involved in the navigation and geographic orientation of a variety of animals and bacteria, although many open questions on their formation and functioning remain. In this review, we will analyze what is known about the natural formation of magnetic nanochains, as well as the synthetic protocols to produce them in the laboratory, to conclude with an overview of medical applications and an outlook on future opportunities in this exciting research field.
Keywords: biocompass; biomineralization; magnetic assembly; magnetic navigation; magnetite; magnetoreception; magnetosome chains; magnetotactic bacteria; single domain particles; superparamagnetic iron oxide nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

