Effective Osteogenic Priming of Mesenchymal Stem Cells through LNA-ASOs-Mediated Sfrp1 Gene Silencing
- PMID: 34452242
- PMCID: PMC8398380
- DOI: 10.3390/pharmaceutics13081277
Effective Osteogenic Priming of Mesenchymal Stem Cells through LNA-ASOs-Mediated Sfrp1 Gene Silencing
Abstract
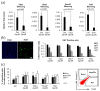
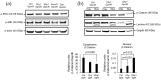
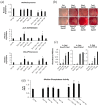
Mesenchymal stem cell (MSC) transplantation has emerged as a promising approach for bone regeneration. Importantly, the beneficial effects of MSCs can be improved by modulating the expression levels of specific genes to stimulate MSC osteogenic differentiation. We have previously shown that Smurf1 silencing by using Locked Nucleic Acid-Antisense Oligonucleotides, in combination with a scaffold that sustainably releases low doses of BMP-2, was able to increase the osteogenic potential of MSCs in the presence of BMP-2 doses significantly smaller than those currently used in the clinic. This would potentially allow an important reduction in this protein in MSs-based treatments, and thus of the side effects linked to its administration. We have further improved this system by specifically targeting the Wnt pathway modulator Sfrp1. This approach not only increases MSC bone regeneration efficiency, but is also able to induce osteogenic differentiation in osteoporotic human MSCs, bypassing the need for BMP-2 induction, underscoring the regenerative potential of this system. Achieving successful osteogenesis with the sole use of LNA-ASOs, without the need of administering pro-osteogenic factors such as BMP-2, would not only reduce the cost of treatments, but would also open the possibility of targeting these LNA-ASOs specifically to MSCs in the bone marrow, allowing us to treat systemic bone loss such as that associated with osteoporosis.
Keywords: BMP; GapmeR; LNA-ASO; Sfrp1; bone regeneration; mesenchymal stem cells; osteogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- He X., Dziak R., Yuan X., Mao K., Genco R., Swihart M., Sarkar D., Li C., Wang C., Lu L., et al. BMP2 genetically engineered MSCs and EPCs promote vascularized bone regeneration in rat critical-sized calvarial bone defects. PLoS ONE. 2013;8:e60473. doi: 10.1371/journal.pone.0060473. - DOI - PMC - PubMed
-
- Rodriguez-Evora M., Garcia-Pizarro E., del Rosario C., Perez-Lopez J., Reyes R., Delgado A., Rodriguez-Rey J.C., Evora C. Smurf1 knocked-down, mesenchymal stem cells and BMP-2 in an electrospun system for bone regeneration. Biomacromolecules. 2014;15:1311–1322. doi: 10.1021/bm401854d. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

