Thermostable and Long-Circulating Albumin-Conjugated Arthrobacter globiformis Urate Oxidase
- PMID: 34452259
- PMCID: PMC8400835
- DOI: 10.3390/pharmaceutics13081298
Thermostable and Long-Circulating Albumin-Conjugated Arthrobacter globiformis Urate Oxidase
Abstract
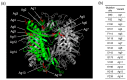
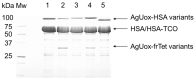
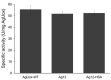
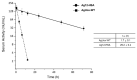
Urate oxidase derived from Aspergillus flavus has been investigated as a treatment for tumor lysis syndrome, hyperuricemia, and gout. However, its long-term use is limited owing to potential immunogenicity, low thermostability, and short circulation time in vivo. Recently, urate oxidase isolated from Arthrobacter globiformis (AgUox) has been reported to be thermostable and less immunogenic than the Aspergillus-derived urate oxidase. Conjugation of human serum albumin (HSA) to therapeutic proteins has become a promising strategy to prolong circulation time in vivo. To develop a thermostable and long-circulating urate oxidase, we investigated the site-specific conjugation of HSA to AgUox based on site-specific incorporation of a clickable non-natural amino acid (frTet) and an inverse electron demand Diels-Alder reaction. We selected 14 sites for frTet incorporation using the ROSETTA design, a computational stability prediction program, among which AgUox containing frTet at position 196 (Ag12) exhibited enzymatic activity and thermostability comparable to those of wild-type AgUox. Furthermore, Ag12 exhibited a high HSA conjugation yield without compromising the enzymatic activity, generating well-defined HSA-conjugated AgUox (Ag12-HSA). In mice, the serum half-life of Ag12-HSA was approximately 29 h, which was roughly 17-fold longer than that of wild-type AgUox. Altogether, this novel formulated AgUox may hold enhanced therapeutic efficacy for several diseases.
Keywords: Arthrobacter globiformis; gout; half-life extension; inverse electron demand Diels-Alder reaction; site-specific albumin conjugation; thermostability; urate oxidase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
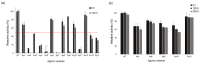


Similar articles
-
Chemical Modification of Cysteine with 3-Arylpropriolonitrile Improves the In Vivo Stability of Albumin-Conjugated Urate Oxidase Therapeutic Protein.Biomedicines. 2021 Sep 27;9(10):1334. doi: 10.3390/biomedicines9101334. Biomedicines. 2021. PMID: 34680451 Free PMC article.
-
Multivalent Albumin-Neonatal Fc Receptor Interactions Mediate a Prominent Extension of the Serum Half-Life of a Therapeutic Protein.Mol Pharm. 2021 Jun 7;18(6):2397-2405. doi: 10.1021/acs.molpharmaceut.1c00231. Epub 2021 May 13. Mol Pharm. 2021. PMID: 33983743
-
In vivo study of newly developed albumin-conjugated urate oxidase for gout treatment.Arthritis Res Ther. 2023 Dec 18;25(1):247. doi: 10.1186/s13075-023-03231-3. Arthritis Res Ther. 2023. PMID: 38111075 Free PMC article.
-
Rasburicase represents a new tool for hyperuricemia in tumor lysis syndrome and in gout.Int J Med Sci. 2007 Mar 2;4(2):83-93. doi: 10.7150/ijms.4.83. Int J Med Sci. 2007. PMID: 17396159 Free PMC article. Review.
-
Recent approaches to gout drug discovery: an update.Expert Opin Drug Discov. 2020 Aug;15(8):943-954. doi: 10.1080/17460441.2020.1755251. Epub 2020 Apr 24. Expert Opin Drug Discov. 2020. PMID: 32329387 Review.
Cited by
-
Data-Driven Technology Roadmaps to Identify Potential Technology Opportunities for Hyperuricemia Drugs.Pharmaceuticals (Basel). 2022 Nov 3;15(11):1357. doi: 10.3390/ph15111357. Pharmaceuticals (Basel). 2022. PMID: 36355529 Free PMC article.
-
Enhanced therapeutic potential of antibody fragment via IEDDA-mediated site-specific albumin conjugation.J Biol Eng. 2024 Apr 4;18(1):23. doi: 10.1186/s13036-024-00418-3. J Biol Eng. 2024. PMID: 38576037 Free PMC article.
-
Chemical Modification of Cysteine with 3-Arylpropriolonitrile Improves the In Vivo Stability of Albumin-Conjugated Urate Oxidase Therapeutic Protein.Biomedicines. 2021 Sep 27;9(10):1334. doi: 10.3390/biomedicines9101334. Biomedicines. 2021. PMID: 34680451 Free PMC article.
-
AlbuCatcher for Long-Acting Therapeutics.ACS Omega. 2024 May 13;9(21):22990-23000. doi: 10.1021/acsomega.4c02303. eCollection 2024 May 28. ACS Omega. 2024. PMID: 38826564 Free PMC article.
References
-
- Harris M.D., Siegel L.B., Alloway J.A. Gout and Hyperuricemia. Am. Fam. Physician. 1999;59:925. - PubMed
-
- Nyborg A.C., Ward C., Zacco A., Chacko B., Grinberg L., Geoghegan J.C., Bean R., Wendeler M., Bartnik F., O’Connor E., et al. A therapeutic uricase with reduced immunogenicity risk and improved development properties. PLoS ONE. 2016;11:e0167935. doi: 10.1371/journal.pone.0167935. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

