3-Indoleacetonitrile Is Highly Effective in Treating Influenza A Virus Infection In Vitro and In Vivo
- PMID: 34452298
- PMCID: PMC8402863
- DOI: 10.3390/v13081433
3-Indoleacetonitrile Is Highly Effective in Treating Influenza A Virus Infection In Vitro and In Vivo
Abstract
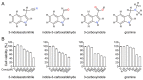
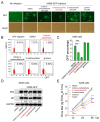
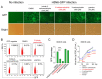
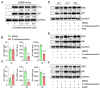
Influenza A viruses are serious zoonotic pathogens that continuously cause pandemics in several animal hosts, including birds, pigs, and humans. Indole derivatives containing an indole core framework have been extensively studied and developed to prevent and/or treat viral infection. This study evaluated the anti-influenza activity of several indole derivatives, including 3-indoleacetonitrile, indole-3-carboxaldehyde, 3-carboxyindole, and gramine, in A549 and MDCK cells. Among these compounds, 3-indoleacetonitrile exerts profound antiviral activity against a broad spectrum of influenza A viruses, as tested in A549 cells. Importantly, in a mouse model, 3-indoleacetonitrile with a non-toxic concentration of 20 mg/kg effectively reduced the mortality and weight loss, diminished lung virus titers, and alleviated lung lesions of mice lethally challenged with A/duck/Hubei/WH18/2015 H5N6 and A/Puerto Rico/8/1934 H1N1 influenza A viruses. The antiviral properties enable the potential use of 3-indoleacetonitrile for the treatment of IAV infection.
Keywords: 3-indoleacetonitrile; antiviral; indole derivatives; indole-3-carboxaldehyde; influenza A virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

