Inhibition of Orbivirus Replication by Fluvastatin and Identification of the Key Elements of the Mevalonate Pathway Involved
- PMID: 34452303
- PMCID: PMC8402872
- DOI: 10.3390/v13081437
Inhibition of Orbivirus Replication by Fluvastatin and Identification of the Key Elements of the Mevalonate Pathway Involved
Abstract
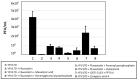
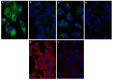
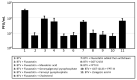
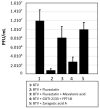
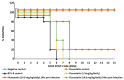
Statin derivatives can inhibit the replication of a range of viruses, including hepatitis C virus (HCV, Hepacivirus), dengue virus (Flavivirus), African swine fever virus (Asfarviridae) and poliovirus (Picornaviridae). We assess the antiviral effect of fluvastatin in cells infected with orbiviruses (bluetongue virus (BTV) and Great Island virus (GIV)). The synthesis of orbivirus outer-capsid protein VP2 (detected by confocal immunofluorescence imaging) was used to assess levels of virus replication, showing a reduction in fluvastatin-treated cells. A reduction in virus titres of ~1.7 log (98%) in fluvastatin-treated cells was detected by a plaque assay. We have previously identified a fourth non-structural protein (NS4) of BTV and GIV, showing that it interacts with lipid droplets in infected cells. Fluvastatin, which inhibits 3-hydroxy 3-methyl glutaryl CoA reductase in the mevalonic acid pathway, disrupts these NS4 interactions. These findings highlight the role of the lipid pathways in orbivirus replication and suggest a greater role for the membrane-enveloped orbivirus particles than previously recognised. Chemical intermediates of the mevalonic acid pathway were used to assess their potential to rescue orbivirus replication. Pre-treatment of IFNAR(-/-) mice with fluvastatin promoted their survival upon challenge with live BTV, although only limited protection was observed.
Keywords: BTV; HMG-CoA reductase; IFNAR(−/−) mice; antiviral; bluetongue virus; fluvastatin; orbivirus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Orbivirus NS4 Proteins Play Multiple Roles to Dampen Cellular Responses.Viruses. 2023 Sep 12;15(9):1908. doi: 10.3390/v15091908. Viruses. 2023. PMID: 37766314 Free PMC article.
-
Fluvastatin Inhibits HMG-CoA Reductase and Prevents Non-Small Cell Lung Carcinogenesis.Cancer Prev Res (Phila). 2019 Dec;12(12):837-848. doi: 10.1158/1940-6207.CAPR-19-0211. Epub 2019 Sep 25. Cancer Prev Res (Phila). 2019. PMID: 31554629
-
Interferon α/β receptor knockout mice as a model to study bluetongue virus infection.Virus Res. 2014 Mar;182:35-42. doi: 10.1016/j.virusres.2013.09.038. Epub 2013 Oct 4. Virus Res. 2014. PMID: 24100234 Review.
-
Inhibition of Orbivirus Replication by Aurintricarboxylic Acid.Int J Mol Sci. 2020 Oct 2;21(19):7294. doi: 10.3390/ijms21197294. Int J Mol Sci. 2020. PMID: 33023235 Free PMC article.
-
[Reverse genetics systems for orbiviruses reveal the essential mechanisms in their replication].Uirusu. 2014;64(2):203-12. doi: 10.2222/jsv.64.203. Uirusu. 2014. PMID: 26437842 Review. Japanese.
Cited by
-
Orbivirus NS4 Proteins Play Multiple Roles to Dampen Cellular Responses.Viruses. 2023 Sep 12;15(9):1908. doi: 10.3390/v15091908. Viruses. 2023. PMID: 37766314 Free PMC article.
-
Redirecting Imipramine against Bluetongue Virus Infection: Insights from a Genome-wide Haploid Screening Study.Pathogens. 2022 May 22;11(5):602. doi: 10.3390/pathogens11050602. Pathogens. 2022. PMID: 35631123 Free PMC article.
-
Do Statins Affect Viral Infections Encountered by International Travelers?Trop Med Infect Dis. 2025 Mar 11;10(3):73. doi: 10.3390/tropicalmed10030073. Trop Med Infect Dis. 2025. PMID: 40137827 Free PMC article. Review.
-
Increased Clinical Signs and Mortality in IFNAR(-/-) Mice Immunised with the Bluetongue Virus Outer-Capsid Proteins VP2 or VP5, after Challenge with an Attenuated Heterologous Serotype.Pathogens. 2023 Apr 15;12(4):602. doi: 10.3390/pathogens12040602. Pathogens. 2023. PMID: 37111488 Free PMC article.
-
The Combined Expression of the Nonstructural Protein NS1 and the N-Terminal Half of NS2 (NS21-180) by ChAdOx1 and MVA Confers Protection against Clinical Disease in Sheep upon Bluetongue Virus Challenge.J Virol. 2022 Feb 9;96(3):e0161421. doi: 10.1128/JVI.01614-21. Epub 2021 Nov 17. J Virol. 2022. PMID: 34787454 Free PMC article.
References
-
- Attoui H., Mertens P.P.C., Becnel J., Belaganahalli M., Bergoin M., Brussaard C.P., Chappell J.D., Ciarlet M., del Vas M., Dermody T.S., et al. Virus Taxonomy. The Ninth Report of the International Committee on Taxonomy of Viruses. Elsevier-Academic Press; London, UK: 2012. Reoviridae.
-
- Attoui H., Mendez-Lopez M.R., Rao S., Hurtado-Alendes A., Lizaraso-Caparo F., Mohd Jaafar F., Samuel A.R., Belhouchet M., Pritchard L.I., Melville L., et al. Peruvian horse sickness virus and Yunnan orbivirus, isolated from vertebrates and mosquitoes in Peru and Australia. Virology. 2009;394:298–310. doi: 10.1016/j.virol.2009.08.032. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

