Efficient Transendothelial Migration of Latently HIV-1-Infected Cells
- PMID: 34452453
- PMCID: PMC8402846
- DOI: 10.3390/v13081589
Efficient Transendothelial Migration of Latently HIV-1-Infected Cells
Abstract
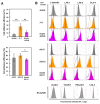
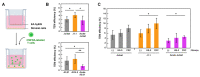
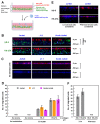
A small fraction of HIV-1-infected T cells forms populations of latently infected cells when they are a naive T-cell subset or in transit to a resting memory state. Latently HIV-1-infected cells reside in lymphoid tissues and serve as viral reservoirs. However, whether they systemically recirculate in the body and re-enter the lymphoid nodes are unknown. Here, we employed two in-vitro cell coculture systems mimicking the lymphatic endothelium in lymph nodes and investigated the homing potential, specifically the transendothelial migration (TEM), of two latently HIV-1-infected cell lines (J1.1 and ACH-2). In trans-well coculture systems, J1.1 and ACH-2 showed higher TEM efficiencies than their parental uninfected and acutely infected cells. The efficiency of TEM was enhanced by the presence of stromal cells, such as HS-5 and fibroblastic reticular cells. In an in-vitro reconstituted, three-dimensional coculture system in which stromal cells are embedded in collagen matrices, J1.1 showed slightly higher TEM efficiency in the presence of HS-5. In accordance with these phenotypes, latently infected cells adhered to the endothelial cells more efficiently than uninfected cells. Together, our study showed that latently HIV-1-infected cells enhanced cell adhesion and TEM abilities, suggesting their potential for efficient homing to lymph nodes.
Keywords: HIV-1; latent infection; lymphocyte homing; reconstitution; stromal cells; transmigration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Finzi D., Hermankova M., Pierson T., Carruth L.M., Buck C., Chaisson R.E., Quinn T.C., Chadwick K., Margolick J., Brookmeyer R., et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

