Bioprinted Multi-Cell Type Lung Model for the Study of Viral Inhibitors
- PMID: 34452455
- PMCID: PMC8402746
- DOI: 10.3390/v13081590
Bioprinted Multi-Cell Type Lung Model for the Study of Viral Inhibitors
Abstract
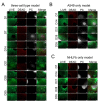
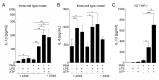
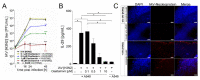
Influenza A virus (IAV) continuously causes epidemics and claims numerous lives every year. The available treatment options are insufficient and the limited pertinence of animal models for human IAV infections is hampering the development of new therapeutics. Bioprinted tissue models support studying pathogenic mechanisms and pathogen-host interactions in a human micro tissue environment. Here, we describe a human lung model, which consisted of a bioprinted base of primary human lung fibroblasts together with monocytic THP-1 cells, on top of which alveolar epithelial A549 cells were printed. Cells were embedded in a hydrogel consisting of alginate, gelatin and collagen. These constructs were kept in long-term culture for 35 days and their viability, expression of specific cell markers and general rheological parameters were analyzed. When the models were challenged with a combination of the bacterial toxins LPS and ATP, a release of the proinflammatory cytokines IL-1β and IL-8 was observed, confirming that the model can generate an immune response. In virus inhibition assays with the bioprinted lung model, the replication of a seasonal IAV strain was restricted by treatment with an antiviral agent in a dose-dependent manner. The printed lung construct provides an alveolar model to investigate pulmonary pathogenic biology and to support development of new therapeutics not only for IAV, but also for other viruses.
Keywords: LPS stimulation; bioprinting; human lung model; influenza A virus.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



Similar articles
-
Anti-Influenza Activity of the Ribonuclease Binase: Cellular Targets Detected by Quantitative Proteomics.Int J Mol Sci. 2020 Nov 5;21(21):8294. doi: 10.3390/ijms21218294. Int J Mol Sci. 2020. PMID: 33167434 Free PMC article.
-
Efficient Inhibition of Avian and Seasonal Influenza A Viruses by a Virus-Specific Dicer-Substrate Small Interfering RNA Swarm in Human Monocyte-Derived Macrophages and Dendritic Cells.J Virol. 2019 Feb 5;93(4):e01916-18. doi: 10.1128/JVI.01916-18. Print 2019 Feb 15. J Virol. 2019. PMID: 30463970 Free PMC article.
-
Cirsimaritin inhibits influenza A virus replication by downregulating the NF-κB signal transduction pathway.Virol J. 2018 May 21;15(1):88. doi: 10.1186/s12985-018-0995-6. Virol J. 2018. PMID: 29783993 Free PMC article.
-
Response Modifiers: Tweaking the Immune Response Against Influenza A Virus.Front Immunol. 2019 Apr 12;10:809. doi: 10.3389/fimmu.2019.00809. eCollection 2019. Front Immunol. 2019. PMID: 31031778 Free PMC article. Review.
-
Modulation of Innate Immune Responses by the Influenza A NS1 and PA-X Proteins.Viruses. 2018 Dec 12;10(12):708. doi: 10.3390/v10120708. Viruses. 2018. PMID: 30545063 Free PMC article. Review.
Cited by
-
Experimental Study on Compatibility of Human Bronchial Epithelial Cells in Collagen-Alginate Bioink for 3D Printing.Bioengineering (Basel). 2024 Aug 23;11(9):862. doi: 10.3390/bioengineering11090862. Bioengineering (Basel). 2024. PMID: 39329604 Free PMC article.
-
Development of a Nanoparticle System for Controlled Release in Bioprinted Respiratory Scaffolds.J Funct Biomater. 2024 Jan 12;15(1):20. doi: 10.3390/jfb15010020. J Funct Biomater. 2024. PMID: 38248687 Free PMC article.
-
High-throughput bioprinting of the nasal epithelium using patient-derived nasal epithelial cells.Biofabrication. 2023 Aug 14;15(4):044103. doi: 10.1088/1758-5090/aced23. Biofabrication. 2023. PMID: 37536321 Free PMC article.
-
3D printing of bio-instructive materials: Toward directing the cell.Bioact Mater. 2022 Apr 23;19:292-327. doi: 10.1016/j.bioactmat.2022.04.008. eCollection 2023 Jan. Bioact Mater. 2022. PMID: 35574057 Free PMC article.
-
3D Tumor Models in Urology.Int J Mol Sci. 2023 Mar 25;24(7):6232. doi: 10.3390/ijms24076232. Int J Mol Sci. 2023. PMID: 37047203 Free PMC article. Review.
References
-
- Troeger C., Forouzanfar M., Rao P.C., Khalil I., Brown A., Swartz S., Fullman N., Mosser J., Thompson R.L., Reiner R.C., et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory tract infections in 195 countries: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect. Dis. 2017;17:1133–1161. doi: 10.1016/S1473-3099(17)30396-1. - DOI - PMC - PubMed
-
- Osterlund P., Pirhonen J., Ikonen N., Ronkko E., Strengell M., Makela S.M., Broman M., Hamming O.J., Hartmann R., Ziegler T., et al. Pandemic H1N1 2009 Influenza A Virus Induces Weak Cytokine Responses in Human Macrophages and Dendritic Cells and Is Highly Sensitive to the Antiviral Actions of Interferons. J. Virol. 2010;84:1414–1422. doi: 10.1128/JVI.01619-09. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

