A Novel Frameshifting Inhibitor Having Antiviral Activity against Zoonotic Coronaviruses
- PMID: 34452503
- PMCID: PMC8402677
- DOI: 10.3390/v13081639
A Novel Frameshifting Inhibitor Having Antiviral Activity against Zoonotic Coronaviruses
Abstract
Recent outbreaks of zoonotic coronaviruses, such as Middle East respiratory syndrome coronavirus (MERS-CoV) and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), have caused tremendous casualties and great economic shock. Although some repurposed drugs have shown potential therapeutic efficacy in clinical trials, specific therapeutic agents targeting coronaviruses have not yet been developed. During coronavirus replication, a replicase gene cluster, including RNA-dependent RNA polymerase (RdRp), is alternatively translated via a process called -1 programmed ribosomal frameshift (-1 PRF) by an RNA pseudoknot structure encoded in viral RNAs. The coronavirus frameshifting has been identified previously as a target for antiviral therapy. In this study, the frameshifting efficiencies of MERS-CoV, SARS-CoV and SARS-CoV-2 were determined using an in vitro -1 PRF assay system. Our group has searched approximately 9689 small molecules to identify potential -1 PRF inhibitors. Herein, we found that a novel compound, 2-(5-acetylthiophen-2yl)furo[2,3-b]quinoline (KCB261770), inhibits the frameshifting of MERS-CoV and effectively suppresses viral propagation in MERS-CoV-infected cells. The inhibitory effects of 87 derivatives of furo[2,3-b]quinolines were also examined showing less prominent inhibitory effect when compared to compound KCB261770. We demonstrated that KCB261770 inhibits the frameshifting without suppressing cap-dependent translation. Furthermore, this compound was able to inhibit the frameshifting, to some extent, of SARS-CoV and SARS-CoV-2. Therefore, the novel compound 2-(5-acetylthiophen-2yl)furo[2,3-b]quinoline may serve as a promising drug candidate to interfere with pan-coronavirus frameshifting.
Keywords: MERS-CoV; SARS-CoV-2; coronavirus; frameshifting; inhibitor.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
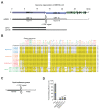
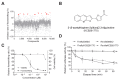

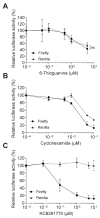
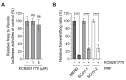
Similar articles
-
Identifying Inhibitors of -1 Programmed Ribosomal Frameshifting in a Broad Spectrum of Coronaviruses.Viruses. 2022 Jan 18;14(2):177. doi: 10.3390/v14020177. Viruses. 2022. PMID: 35215770 Free PMC article.
-
Restriction of SARS-CoV-2 replication by targeting programmed -1 ribosomal frameshifting.Proc Natl Acad Sci U S A. 2021 Jun 29;118(26):e2023051118. doi: 10.1073/pnas.2023051118. Proc Natl Acad Sci U S A. 2021. PMID: 34185680 Free PMC article.
-
Abelson Kinase Inhibitors Are Potent Inhibitors of Severe Acute Respiratory Syndrome Coronavirus and Middle East Respiratory Syndrome Coronavirus Fusion.J Virol. 2016 Sep 12;90(19):8924-33. doi: 10.1128/JVI.01429-16. Print 2016 Oct 1. J Virol. 2016. PMID: 27466418 Free PMC article.
-
Broad-spectrum coronavirus antiviral drug discovery.Expert Opin Drug Discov. 2019 Apr;14(4):397-412. doi: 10.1080/17460441.2019.1581171. Epub 2019 Mar 8. Expert Opin Drug Discov. 2019. PMID: 30849247 Free PMC article. Review.
-
Targeting Ribosomal Frameshifting as an Antiviral Strategy: From HIV-1 to SARS-CoV-2.Acc Chem Res. 2021 Sep 7;54(17):3349-3361. doi: 10.1021/acs.accounts.1c00316. Epub 2021 Aug 17. Acc Chem Res. 2021. PMID: 34403258 Review.
Cited by
-
Identifying Inhibitors of -1 Programmed Ribosomal Frameshifting in a Broad Spectrum of Coronaviruses.Viruses. 2022 Jan 18;14(2):177. doi: 10.3390/v14020177. Viruses. 2022. PMID: 35215770 Free PMC article.
-
Shapify: Paths to SARS-CoV-2 frameshifting pseudoknot.PLoS Comput Biol. 2023 Feb 28;19(2):e1010922. doi: 10.1371/journal.pcbi.1010922. eCollection 2023 Feb. PLoS Comput Biol. 2023. PMID: 36854032 Free PMC article.
-
Modelling the structures of frameshift-stimulatory pseudoknots from representative bat coronaviruses.PLoS Comput Biol. 2023 May 19;19(5):e1011124. doi: 10.1371/journal.pcbi.1011124. eCollection 2023 May. PLoS Comput Biol. 2023. PMID: 37205708 Free PMC article.
-
Tying the knot: Unraveling the intricacies of the coronavirus frameshift pseudoknot.PLoS Comput Biol. 2024 May 7;20(5):e1011787. doi: 10.1371/journal.pcbi.1011787. eCollection 2024 May. PLoS Comput Biol. 2024. PMID: 38713726 Free PMC article.
-
Distinct Molecular Mechanisms Characterizing Pathogenesis of SARS-CoV-2.J Microbiol Biotechnol. 2022 Sep 28;32(9):1073-1085. doi: 10.4014/jmb.2206.06064. Epub 2022 Aug 23. J Microbiol Biotechnol. 2022. PMID: 36039385 Free PMC article. Review.
References
-
- Coronavirus disease (COVID-19) Weekly Epidemiological Update and Weekly Operational Update. World Health Organization; Geneva, Switzerland: 2021.
-
- WHO . MERS Situation Update, March 2021. World Health Organization; Geneva, Switzerland: 2021.
-
- de Groot R.J., Baker S.C., Baric R.S., Brown C.S., Drosten C., Enjuanes L., Fouchier R.A., Galiano M., Gorbalenya A.E., Memish Z.A., et al. Middle East respiratory syndrome coronavirus (MERS-CoV): Announcement of the Coronavirus Study Group. J. Virol. 2013;87:7790–7792. doi: 10.1128/JVI.01244-13. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

