Large-Scale International Validation of an Indirect ELISA Based on Recombinant Nucleocapsid Protein of Rift Valley Fever Virus for the Detection of IgG Antibody in Domestic Ruminants
- PMID: 34452515
- PMCID: PMC8402881
- DOI: 10.3390/v13081651
Large-Scale International Validation of an Indirect ELISA Based on Recombinant Nucleocapsid Protein of Rift Valley Fever Virus for the Detection of IgG Antibody in Domestic Ruminants
Abstract
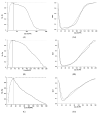
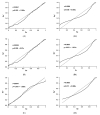
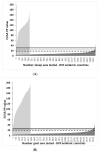
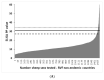
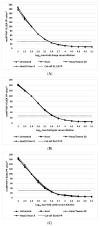
Diagnostic performance of an indirect enzyme-linked immunosorbent assay (I-ELISA) based on a recombinant nucleocapsid protein (rNP) of the Rift Valley fever virus (RVFV) was validated for the detection of the IgG antibody in sheep (n = 3367), goat (n = 2632), and cattle (n = 3819) sera. Validation data sets were dichotomized according to the results of a virus neutralization test in sera obtained from RVF-endemic (Burkina Faso, Democratic Republic of Congo, Mozambique, Senegal, Uganda, and Yemen) and RVF-free countries (France, Poland, and the USA). Cut-off values were defined using the two-graph receiver operating characteristic analysis. Estimates of the diagnostic specificity of the RVFV rNP I-ELISA in animals from RVF-endemic countries ranged from 98.6% (cattle) to 99.5% (sheep) while in those originating from RVF-free countries, they ranged from 97.7% (sheep) to 98.1% (goats). Estimates of the diagnostic sensitivity in ruminants from RVF-endemic countries ranged from 90.7% (cattle) to 100% (goats). The results of this large-scale international validation study demonstrate the high diagnostic accuracy of the RVFV rNP I-ELISA. Standard incubation and inactivation procedures evaluated did not have an adverse effect on the detectable levels of the anti-RVFV IgG in ruminant sera and thus, together with recombinant antigen-based I-ELISA, provide a simple, safe, and robust diagnostic platform that can be automated and carried out outside expensive bio-containment facilities. These advantages are particularly important for less-resourced countries where there is a need to accelerate and improve RVF surveillance and research on epidemiology as well as to advance disease control measures.
Keywords: IgG antibody; Rift Valley fever virus; diagnostic accuracy; domestic ruminants; enzyme-linked immunosorbent assay; recombinant nucleocapsid; validation.
Conflict of interest statement
The authors declare no conflict of interest. The funder had no role in the design of the study; the collection, analyses, or interpretation of data; in the writing of the manuscript; or the decision to publish the results.
Figures


References
-
- Paweska J.T., Jansen van Vuren P. Rift Valley fever virus: A virus with potential for global emergence. In: Johnson N., editor. The Role of Animals in Emerging Viral Diseases. Elsevier; Amsterdam, The Netherlands: Academic Press; Cambridge, MA, USA: 2013. pp. 169–200.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

