The Microvillar and Solitary Chemosensory Cells as the Novel Targets of Infection of SARS-CoV-2 in Syrian Golden Hamsters
- PMID: 34452517
- PMCID: PMC8402700
- DOI: 10.3390/v13081653
The Microvillar and Solitary Chemosensory Cells as the Novel Targets of Infection of SARS-CoV-2 in Syrian Golden Hamsters
Abstract
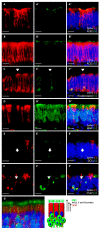
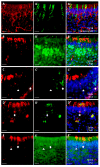
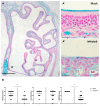
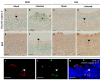
Patients infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of coronavirus disease 2019, suffer from respiratory and non-respiratory symptoms. Among these symptoms, the loss of smell has attracted considerable attention. The objectives of this study were to determine which cells are infected, what happens in the olfactory system after viral infection, and how these pathologic changes contribute to olfactory loss. For this purpose, Syrian golden hamsters were used. First, we verified the olfactory structures in the nasal cavity of Syrian golden hamsters, namely the main olfactory epithelium, the vomeronasal organ, and their cellular components. Second, we found angiotensin-converting enzyme 2 expression, a receptor protein of SARS-CoV-2, in both structures and infections of supporting, microvillar, and solitary chemosensory cells. Third, we observed pathological changes in the infected epithelium, including reduced thickness of the mucus layer, detached epithelia, indistinct layers of epithelia, infiltration of inflammatory cells, and apoptotic cells in the overall layers. We concluded that a structurally and functionally altered microenvironment influences olfactory function. We observed the regeneration of the damaged epithelium, and found multilayers of basal cells, indicating that they were activated and proliferating to reconstitute the injured epithelium.
Keywords: SARS-CoV-2; microvillar cell; olfactory system; regeneration; solitary chemosensory cell.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




Similar articles
-
Regeneration Profiles of Olfactory Epithelium after SARS-CoV-2 Infection in Golden Syrian Hamsters.ACS Chem Neurosci. 2021 Feb 17;12(4):589-595. doi: 10.1021/acschemneuro.0c00649. Epub 2021 Feb 1. ACS Chem Neurosci. 2021. PMID: 33522795 Free PMC article.
-
Massive transient damage of the olfactory epithelium associated with infection of sustentacular cells by SARS-CoV-2 in golden Syrian hamsters.Brain Behav Immun. 2020 Oct;89:579-586. doi: 10.1016/j.bbi.2020.06.032. Epub 2020 Jul 3. Brain Behav Immun. 2020. PMID: 32629042 Free PMC article.
-
COVID-19 Anosmia: High Prevalence, Plural Neuropathogenic Mechanisms, and Scarce Neurotropism of SARS-CoV-2?Viruses. 2021 Nov 4;13(11):2225. doi: 10.3390/v13112225. Viruses. 2021. PMID: 34835030 Free PMC article. Review.
-
Severe Acute Respiratory Syndrome Coronavirus 2 Infects and Damages the Mature and Immature Olfactory Sensory Neurons of Hamsters.Clin Infect Dis. 2021 Jul 15;73(2):e503-e512. doi: 10.1093/cid/ciaa995. Clin Infect Dis. 2021. PMID: 32667973 Free PMC article.
-
Receptors Involved in COVID-19-Related Anosmia: An Update on the Pathophysiology and the Mechanistic Aspects.Int J Mol Sci. 2024 Aug 5;25(15):8527. doi: 10.3390/ijms25158527. Int J Mol Sci. 2024. PMID: 39126095 Free PMC article. Review.
Cited by
-
Immune responses in the injured olfactory and gustatory systems: a role in olfactory receptor neuron and taste bud regeneration?Chem Senses. 2022 Jan 1;47:bjac024. doi: 10.1093/chemse/bjac024. Chem Senses. 2022. PMID: 36152297 Free PMC article. Review.
-
Olfactory dysfunction in COVID-19: new insights into the underlying mechanisms.Trends Neurosci. 2023 Jan;46(1):75-90. doi: 10.1016/j.tins.2022.11.003. Epub 2022 Nov 16. Trends Neurosci. 2023. PMID: 36470705 Free PMC article. Review.
-
Neuroimmune recognition and regulation in the respiratory system.Eur Respir Rev. 2024 Jun 26;33(172):240008. doi: 10.1183/16000617.0008-2024. Print 2024 Apr. Eur Respir Rev. 2024. PMID: 38925790 Free PMC article. Review.
-
Evidence for the spread of SARS-CoV-2 and olfactory cell lineage impairment in close-contact infection Syrian hamster models.Front Cell Infect Microbiol. 2022 Oct 21;12:1019723. doi: 10.3389/fcimb.2022.1019723. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36339331 Free PMC article.
-
Neutrophils play a major role in the destruction of the olfactory epithelium during SARS-CoV-2 infection in hamsters.Cell Mol Life Sci. 2022 Dec 3;79(12):616. doi: 10.1007/s00018-022-04643-1. Cell Mol Life Sci. 2022. PMID: 36460750 Free PMC article.
References
-
- Centers for Disease Control and Prevention, Symptom of Coronavirus. [(accessed on 22 February 2021)]; Available online: http://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

