Protective Effects of Astodrimer Sodium 1% Nasal Spray Formulation against SARS-CoV-2 Nasal Challenge in K18-hACE2 Mice
- PMID: 34452519
- PMCID: PMC8402925
- DOI: 10.3390/v13081656
Protective Effects of Astodrimer Sodium 1% Nasal Spray Formulation against SARS-CoV-2 Nasal Challenge in K18-hACE2 Mice
Abstract
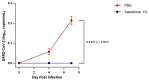
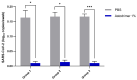
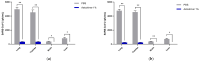
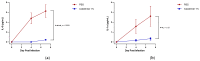
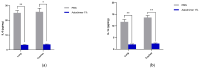
Strategies to combat COVID-19 require multiple ways to protect vulnerable people from infection. SARS-CoV-2 is an airborne pathogen and the nasal cavity is a primary target of infection. The K18-hACE2 mouse model was used to investigate the anti-SARS-CoV-2 efficacy of astodrimer sodium formulated in a mucoadhesive nasal spray. Animals received astodrimer sodium 1% nasal spray or PBS intranasally, or intranasally and intratracheally, for 7 days, and they were infected intranasally with SARS-CoV-2 after the first product administration on Day 0. Another group was infected intranasally with SARS-CoV-2 that had been pre-incubated with astodrimer sodium 1% nasal spray or PBS for 60 min before the neutralisation of test product activity. Astodrimer sodium 1% significantly reduced the viral genome copies (>99.9%) and the infectious virus (~95%) in the lung and trachea vs. PBS. The pre-incubation of SARS-CoV-2 with astodrimer sodium 1% resulted in a significant reduction in the viral genome copies (>99.9%) and the infectious virus (>99%) in the lung and trachea, and the infectious virus was not detected in the brain or liver. Astodrimer sodium 1% resulted in a significant reduction of viral genome copies in nasal secretions vs. PBS on Day 7 post-infection. A reduction in the viral shedding from the nasal cavity may result in lower virus transmission rates. Viraemia was low or undetectable in animals treated with astodrimer sodium 1% or infected with treated virus, correlating with the lack of detectable viral replication in the liver. Similarly, low virus replication in the nasal cavity after treatment with astodrimer sodium 1% potentially protected the brain from infection. Astodrimer sodium 1% significantly reduced the pro-inflammatory cytokines IL-6, IL-1α, IL-1β, TNFα and TGFβ and the chemokine MCP-1 in the serum, lung and trachea vs. PBS. Astodrimer sodium 1% nasal spray blocked or reduced SARS-CoV-2 replication and its sequelae in K18-hACE2 mice. These data indicate a potential role for the product in preventing SARS-CoV-2 infection or for reducing the severity of COVID-19.
Keywords: COVID-19; SARS-CoV-2; SPL7013; animal model; antiviral; astodrimer; dendrimer; nasal.
Conflict of interest statement
The authors M.D.B. and P.A.G. declare no conflict of interest. The authors J.R.A.P. and G.P.H. are paid employees of Starpharma Pty Ltd. The authors C.A.L. and A.C. are paid consultants to Starpharma Pty Ltd. Starpharma Pty Ltd. was responsible for the study design, the interpretation of data, the writing of the manuscript and the decision to submit the article for publication. The MRFF BTB funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures





References
-
- Tyssen D., Henderson S.A., Johnson A., Sterjovski J., Moore K., La J., Zanin M., Sonza S., Karellas P., Giannis M.P., et al. Structure Activity Relationship of Dendrimer Microbicides with Dual Action Antiviral Activity. PLoS ONE. 2010;5:e12309. doi: 10.1371/journal.pone.0012309. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

