Equine Influenza Virus and Vaccines
- PMID: 34452521
- PMCID: PMC8402878
- DOI: 10.3390/v13081657
Equine Influenza Virus and Vaccines
Abstract
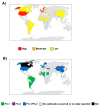
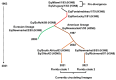
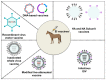
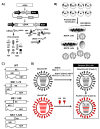
Equine influenza virus (EIV) is a constantly evolving viral pathogen that is responsible for yearly outbreaks of respiratory disease in horses termed equine influenza (EI). There is currently no evidence of circulation of the original H7N7 strain of EIV worldwide; however, the EIV H3N8 strain, which was first isolated in the early 1960s, remains a major threat to most of the world's horse populations. It can also infect dogs. The ability of EIV to constantly accumulate mutations in its antibody-binding sites enables it to evade host protective immunity, making it a successful viral pathogen. Clinical and virological protection against EIV is achieved by stimulation of strong cellular and humoral immunity in vaccinated horses. However, despite EI vaccine updates over the years, EIV remains relevant, because the protective effects of vaccines decay and permit subclinical infections that facilitate transmission into susceptible populations. In this review, we describe how the evolution of EIV drives repeated EI outbreaks even in horse populations with supposedly high vaccination coverage. Next, we discuss the approaches employed to develop efficacious EI vaccines for commercial use and the existing system for recommendations on updating vaccines based on available clinical and virological data to improve protective immunity in vaccinated horse populations. Understanding how EIV biology can be better harnessed to improve EI vaccines is central to controlling EI.
Keywords: H3N8; adaptive immunity; cellular immunity; equine influenza; equine influenza vaccine; equine influenza virus; experimental infection; humoral immunity; surveillance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Timoney P.J. Equine Infectious Diseases. 2nd ed. Elsevier; Amsterdam, The Netherlands: 2014. Infectious diseases and international movement of horses; pp. 544–551.e1.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

