Efficacy of Unsupervised Self-Collected Mid-Turbinate FLOQSwabs for the Diagnosis of Coronavirus Disease 2019 (COVID-19)
- PMID: 34452527
- PMCID: PMC8402664
- DOI: 10.3390/v13081663
Efficacy of Unsupervised Self-Collected Mid-Turbinate FLOQSwabs for the Diagnosis of Coronavirus Disease 2019 (COVID-19)
Abstract
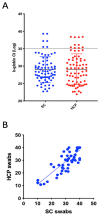
The Global Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) pandemic has resulted in explosive patterns of transmission in most countries. Nasopharyngeal swabs were the specimen's collection tools recommended for the diagnosis of SARS-CoV-2 infection, and for monitoring infection outbreaks in communities. Our objective was to report the quality and efficacy of unsupervised self-collected mid turbinate "dry FLOQSwabs" (MT FLOQSwabs) (56380CS01, Copan). There were 111 specimens collected for the study: 36 by health care personnel, from themselves, to verify the quality and efficacy of mid-turbinate swabs; 75 to compare and assess the diagnostic performance, among health care personnel, of nasopharyngeal swabs and self-collected mid-turbinate FLOQSwabs. A collection of 51 specimens was enrolled to define the efficacy of the Testami program (validation). Our analyses demonstrate that self-collected mid-turbinate dry swabs ensure an accuracy of 97.3%, as compared to the standard nasopharyngeal swabs collected by health care workers. Furthermore, the mid-turbinate FLOQSwabs can be stored without medium for six days at room temperature without affecting the molecular diagnosis of the SARS-CoV-2 virus infection. Self-collection of diagnostic specimens at home could offer an avenue to increase testing availability for SARS-CoV-2 infection without asking people to travel to a clinic or a laboratory, thus reducing people's exposure to infection. Our findings demonstrate that unsupervised self-collection swabs, transported dry, are sensitive, practical and easy-to-use tools and should be considered for diagnosis of SARS-COV-2 and coronavirus disease 2019 (COVID-19) surveillance.
Keywords: SARS-CoV-2; dry swab; surveillance; unsupervised self-collection.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
The accuracy of healthcare worker versus self collected (2-in-1) Oropharyngeal and Bilateral Mid-Turbinate (OPMT) swabs and saliva samples for SARS-CoV-2.PLoS One. 2020 Dec 16;15(12):e0244417. doi: 10.1371/journal.pone.0244417. eCollection 2020. PLoS One. 2020. PMID: 33326503 Free PMC article.
-
Performance of anterior nares and tongue swabs for nucleic acid, Nucleocapsid, and Spike antigen testing for detecting SARS-CoV-2 against nasopharyngeal PCR and viral culture.Int J Infect Dis. 2022 Apr;117:287-294. doi: 10.1016/j.ijid.2022.02.009. Epub 2022 Feb 9. Int J Infect Dis. 2022. PMID: 35149246 Free PMC article.
-
Practical strategies for SARS-CoV-2 RT-PCR testing in resource-constrained settings.Diagn Microbiol Infect Dis. 2021 Oct;101(2):115469. doi: 10.1016/j.diagmicrobio.2021.115469. Epub 2021 Jun 25. Diagn Microbiol Infect Dis. 2021. PMID: 34280773 Free PMC article.
-
Current status of the lateral flow immunoassay for the detection of SARS-CoV-2 in nasopharyngeal swabs.Biochem Med (Zagreb). 2021 Jun 15;31(2):020601. doi: 10.11613/BM.2021.020601. Biochem Med (Zagreb). 2021. PMID: 34140830 Free PMC article. Review.
-
Relative sensitivity of anterior nares and nasopharyngeal swabs for initial detection of SARS-CoV-2 in ambulatory patients: Rapid review and meta-analysis.PLoS One. 2021 Jul 20;16(7):e0254559. doi: 10.1371/journal.pone.0254559. eCollection 2021. PLoS One. 2021. PMID: 34283845 Free PMC article. Review.
Cited by
-
Molecular Diagnosis of COVID-19; Biosafety and Pre-analytical Recommendations.Iran J Pathol. 2023 Summer;18(3):244-256. doi: 10.30699/IJP.2023.1988405.3061. Epub 2023 Jul 16. Iran J Pathol. 2023. PMID: 37942195 Free PMC article. Review.
-
Importance of Adequate qPCR Controls in Infection Control.Diagnostics (Basel). 2021 Dec 16;11(12):2373. doi: 10.3390/diagnostics11122373. Diagnostics (Basel). 2021. PMID: 34943608 Free PMC article.
-
Detection of SARS-CoV-2 RNA by Reverse Transcription-Polymerase Chain Reaction (RT-PCR) on Self-Collected Nasal Swab Compared With Professionally Collected Nasopharyngeal Swab.Cureus. 2022 Jun 3;14(6):e25618. doi: 10.7759/cureus.25618. eCollection 2022 Jun. Cureus. 2022. PMID: 35784954 Free PMC article.
-
The effect of sample site and collection procedure on identification of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2024 Dec 16;12(12):CD014780. doi: 10.1002/14651858.CD014780. Cochrane Database Syst Rev. 2024. PMID: 39679851 Free PMC article.
-
Knowledge of SARS-CoV-2 antigen detection and proper use of rapid diagnostic self-test among Shanghai residents in China.Front Public Health. 2023 Jan 24;11:1036823. doi: 10.3389/fpubh.2023.1036823. eCollection 2023. Front Public Health. 2023. PMID: 36761141 Free PMC article.
References
-
- Smieja M., Castriciano S., Carruthers S., So G., Chong S., Luinstra K., Mahony J.B., Petrich A., Chernesky M., Savarese M., et al. Development and Evaluation of a Flocked Nasal Midturbinate Swab for Self-Collection in Respiratory Virus Infection Diagnostic Testing. J. Clin. Microbiol. 2010;48:3340–3342. doi: 10.1128/JCM.02235-09. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

