3D printing of dual-cell delivery titanium alloy scaffolds for improving osseointegration through enhancing angiogenesis and osteogenesis
- PMID: 34452607
- PMCID: PMC8401189
- DOI: 10.1186/s12891-021-04617-7
3D printing of dual-cell delivery titanium alloy scaffolds for improving osseointegration through enhancing angiogenesis and osteogenesis
Abstract
Background: The repair of large bone defects is a great challenge for orthopedics. Although the development of three-dimensional (3D) printed titanium alloy (Ti6Al4V) implants with optimized the pore structure have effectively promoted the osseointegration. However, due to the biological inertia of Ti6Al4Vsurface and the neglect of angiogenesis, some patients still suffer from postoperative complications such as dislocation or loosening of the prosthesis.
Methods: The purpose of this study was to construct 3D printed porous Ti6Al4V scaffolds filled with bone marrow mesenchymal stem cells (BMSC) and endothelial progenitor cells (EPC) loaded hydrogel and evaluate the efficacy of this composite implants on osteogenesis and angiogenesis, thus promoting osseointegration.
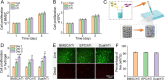
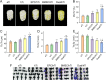
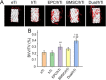
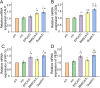
Results: The porosity and pore size of prepared 3D printed porous Ti6Al4V scaffolds were 69.2 ± 0.9 % and 593.4 ± 16.9 μm, respectively, which parameters were beneficial to bone ingrowth and blood vessel formation. The BMSC and EPC filled into the pores of the scaffolds after being encapsulated by hydrogels can maintain high viability. As a cell containing composite implant, BMSC and EPC loaded hydrogel incorporated into 3D printed porous Ti6Al4V scaffolds enhancing osteogenesis and angiogenesis to repair bone defects efficiently. At the transcriptional level, the composite implant up-regulated the expression levels of the osteogenesis-related genes alkaline phosphatase (ALP) and osteocalcin (OCN), and angiogenesis-related genes hypoxia-inducible factor 1 alpha (HIF-1α), and vascular endothelial growth factor (VEGF).
Conclusions: Overall, the strategy of loading porous Ti6Al4V scaffolds to incorporate cells is a promising treatment for improving osseointegration.
Keywords: 3D printed; Angiogenesis; Osseointegration; Osteogenesis; Titanium alloy implant.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Guo Y, Ren L, Xie K, Wang L, Yu BH, Jiang WB, Zhao YH, Hao YQ. Functionalized TiCu/Ti-Cu-N-Coated 3D-Printed Porous Ti6Al4V Scaffold Promotes Bone Regeneration through BMSC Recruitment. Adv Mater Interfaces. 2020;7(6):13. doi: 10.1002/admi.201901632. - DOI
-
- Zhao Y, Li ZH, Jiang YN, Liu H, Feng YB, Wang ZH, Liu H, Wang JC, Yang B, Lin Q. Bioinspired mineral hydrogels as nanocomposite scaffolds for the promotion of osteogenic marker expression and the induction of bone regeneration in osteoporosis. Acta Biomater. 2020;113:614–626. doi: 10.1016/j.actbio.2020.06.024. - DOI - PubMed
-
- Mansoorianfar M, Khataee A, Riahi Z, Shahin K, Asadnia M, Razmjou A, Hojjati-Najafabadi A, Mei CT, Orooji Y, Li DG. Scalable fabrication of tunable titanium nanotubes via sonoelectrochemical process for biomedical applications. Ultrason Sonochem. 2020;64:11. doi: 10.1016/j.ultsonch.2019.104783. - DOI - PubMed
-
- Li S, Li X, Hou W, Nune KC, Misra RDK, Correa-Rodriguez VL, Guo Z, Hao Y, Yang R, Murr LE. Fabrication of open-cellular (porous) titanium alloy implants: osseointegration, vascularization and preliminary human trials. Sci China Mater. 2018;61(4):525–536. doi: 10.1007/s40843-017-9063-6. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

