Sustained oxygenation accelerates diabetic wound healing by promoting epithelialization and angiogenesis and decreasing inflammation
- PMID: 34452918
- PMCID: PMC8397271
- DOI: 10.1126/sciadv.abj0153
Sustained oxygenation accelerates diabetic wound healing by promoting epithelialization and angiogenesis and decreasing inflammation
Abstract
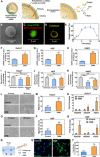
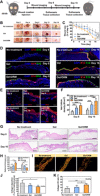
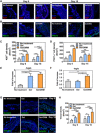
Nonhealing diabetic wounds are common complications for diabetic patients. Because chronic hypoxia prominently delays wound healing, sustained oxygenation to alleviate hypoxia is hypothesized to promote diabetic wound healing. However, sustained oxygenation cannot be achieved by current clinical approaches, including hyperbaric oxygen therapy. Here, we present a sustained oxygenation system consisting of oxygen-release microspheres and a reactive oxygen species (ROS)-scavenging hydrogel. The hydrogel captures the naturally elevated ROS in diabetic wounds, which may be further elevated by the oxygen released from the administered microspheres. The sustained release of oxygen augmented the survival and migration of keratinocytes and dermal fibroblasts, promoted angiogenic growth factor expression and angiogenesis in diabetic wounds, and decreased the proinflammatory cytokine expression. These effects significantly increased the wound closure rate. Our findings demonstrate that sustained oxygenation alone, without using drugs, can heal diabetic wounds.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures





References
-
- Centers for Disease Control and Prevention, in National Diabetes Statistics Report 2020. Estimates of diabetes and its burden in the United States 2020 (Centers for Disease Control and Prevention, 2020), pp. 1–32.
-
- Singh N., Armstrong D. G., Lipsky B. A., Preventing foot ulcers in patients with diabetes. JAMA 293, 217–228 (2005). - PubMed
-
- Gottrup F., Apelqvist J., Present and new techniques and devices in the treatment of DFU: A critical review of evidence. Diabetes Metab. Res. Rev. 28, 64–71 (2012). - PubMed
-
- Falanga V., Wound healing and its impairment in the diabetic foot. Lancet 366, 1736–1743 (2005). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 AR077226/AR/NIAMS NIH HHS/United States
- P30 AR074992/AR/NIAMS NIH HHS/United States
- R01 HL138353/HL/NHLBI NIH HHS/United States
- R01 AR075860/AR/NIAMS NIH HHS/United States
- R01 DK113642/DK/NIDDK NIH HHS/United States
- R01 AR077616/AR/NIAMS NIH HHS/United States
- P30 DK020579/DK/NIDDK NIH HHS/United States
- R01 HD106120/HD/NICHD NIH HHS/United States
- R01 EB022018/EB/NIBIB NIH HHS/United States
- R01 HL138175/HL/NHLBI NIH HHS/United States
- R01 AG056919/AG/NIA NIH HHS/United States
- R01 HL153262/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

