Phase 3 randomised trial of eltrombopag versus standard first-line pharmacological management for newly diagnosed immune thrombocytopaenia (ITP) in children: study protocol
- PMID: 34452956
- PMCID: PMC8404450
- DOI: 10.1136/bmjopen-2020-044885
Phase 3 randomised trial of eltrombopag versus standard first-line pharmacological management for newly diagnosed immune thrombocytopaenia (ITP) in children: study protocol
Abstract
Introduction: Immune thrombocytopaenia (ITP) is an acquired disorder of low platelets and risk of bleeding. Although many children can be observed until spontaneous remission, others require treatment due to bleeding or impact on health-related quality of life. Standard first-line therapies for those who need intervention include corticosteroids, intravenous immunoglobulin and anti-D globulin, though response to these agents may be only transient. Eltrombopag is an oral thrombopoietin receptor agonist approved for children with chronic ITP who have had an insufficient response to corticosteroids, intravenous immunoglobulin or splenectomy. This protocol paper describes an ongoing open-label, randomised trial comparing eltrombopag to standard first-line management in children with newly diagnosed ITP.
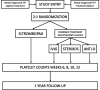
Methods and analysis: Randomised treatment assignment is 2:1 for eltrombopag versus standard first-line management and is stratified by age and by prior treatment. The primary endpoint of the study is platelet response, defined as ≥3 of 4 weeks with platelets >50×109/L during weeks 6-12 of therapy. Secondary outcomes include number of rescue therapies needed during the first 12 weeks, proportion of patients who do not need ongoing treatment at 12 weeks and 6 months, proportion of patients with a treatment response at 1 year, and number of second-line therapies used in weeks 13-52, as well as changes in regulatory T cells, iron studies, bleeding, health-related quality of life and fatigue. A planned sample size of up to 162 randomised paediatric patients will be enrolled over 2 years at 20 sites.
Ethics and dissemination: The study has been approved by the centralised Baylor University Institutional Review Board. The results are expected to be published in 2023.
Trial registration number: NCT03939637.
Keywords: clinical trials; haematology; paediatrics.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: KAS: Research funding: Novartis, Pfizer, Daiichi Sankyo, Alexion; Consultancies: Dova. RFG: Research funding: Novartis, Agios, Pfizer; Consultancies: Agios, Dova. JD: Research funding: Amgen, Novartis; Consultancies: Amgen, Novartis, Dova. EN: Advisory boards: Genentech, NovoNordisk, Novartis; Honoraria: Octapharma; DSMB service: Bayer, ApoPharma, Acceleron, Imara; Consultancies: Pfizer, Celgene. RJK: Speaker: Takeda, Biogen Canada LMT, Octapharma, Pfizer; Consultancies: Agios, Amgen, Hoffman-LaRoche, Takeda, NovoNordisk Canada. CM: Research funding: Novartis. WL: DSMB member: ArQule, Jubliant Draximage. CEN: Research funding: PDSA.