Comprehensive non-invasive prenatal screening for pregnancies with elevated risks of genetic disorders: protocol for a prospective, multicentre study
- PMID: 34452972
- PMCID: PMC8404451
- DOI: 10.1136/bmjopen-2021-053617
Comprehensive non-invasive prenatal screening for pregnancies with elevated risks of genetic disorders: protocol for a prospective, multicentre study
Abstract
Introduction: Chromosomal abnormalities and monogenic disorders account for ~15%-25% of recognisable birth defects. With limited treatment options, preconception and prenatal screening were developed to reduce the incidence of such disorders. Currently, non-invasive prenatal screening (NIPS) for common aneuploidies is implemented worldwide with superiority over conventional serum or sonographic screening approaches. However, the clinical validity for the screening of frequent chromosome segmental copy number variations and monogenic disorders still awaits to be proved.
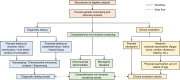
Methods and analysis: This study is a multicentre, prospective study. The participants were recruited from three tertiary hospitals in China starting from 10 April 2021. The study is expected to conclude before 10 October 2022. Pregnant women with abnormal prenatal screening results indicated for invasive prenatal diagnosis or those who decide to terminate their pregnancies due to abnormal ultrasound findings will be evaluated for enrolment. Cell-free DNA extracted from the maternal plasma will be used for an analytically validated comprehensive NIPS test developed by Beijing BioBiggen Technology Co. (Beijing, China). The diagnostic results from prenatal or postnatal specimens as well as the pregnancy outcome data will be collected to examine the clinical sensitivity, specificity, positive and negative predictive values of the test.
Ethics and dissemination: This study was approved by the Obstetrics and Gynecology Hospital of Fudan University (2020-178). Results of this study will be disseminated to public through scientific conferences and a peer-reviewed journal. Written informed consents will be obtained from participants.
Trial registration number: ChiCTR2100045739.
Keywords: antenatal; preventive medicine; reproductive medicine.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: XC, JZ are employees or shareholders of Beijing BioBiggen Technology Co. The other authors declare no conflict of interest.
Figures

References
-
- Brent RL. Environmental causes of human congenital malformations: the pediatrician’s role in dealing with these complex clinical problems caused by a multiplicity of environmental and genetic factors. Pediatrics 2004;113:957–68. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous
