FtsA acts through FtsW to promote cell wall synthesis during cell division in Escherichia coli
- PMID: 34453005
- PMCID: PMC8536321
- DOI: 10.1073/pnas.2107210118
FtsA acts through FtsW to promote cell wall synthesis during cell division in Escherichia coli
Abstract
In Escherichia coli, FtsQLB is required to recruit the essential septal peptidoglycan (sPG) synthase FtsWI to FtsA, which tethers FtsZ filaments to the membrane. The arrival of FtsN switches FtsQLB in the periplasm and FtsA in the cytoplasm from a recruitment role to active forms that synergize to activate FtsWI. Genetic evidence indicates that the active form of FtsQLB has an altered conformation with an exposed domain of FtsL that acts on FtsI to activate FtsW. However, how FtsA contributes to the activation of FtsW is not clear, as it could promote the conformational change in FtsQLB or act directly on FtsW. Here, we show that the overexpression of an activated FtsA (FtsA*) bypasses FtsQ, indicating it can compensate for FtsQ's recruitment function. Consistent with this, FtsA* also rescued FtsL and FtsB mutants deficient in FtsW recruitment. FtsA* also rescued an FtsL mutant unable to deliver the periplasmic signal from FtsN, consistent with FtsA* acting on FtsW. In support of this, an FtsW mutant was isolated that was rescued by an activated FtsQLB but not by FtsA*, indicating it was specifically defective in activation by FtsA. Our results suggest that in response to FtsN, the active form of FtsA acts on FtsW in the cytoplasm and synergizes with the active form of FtsQLB acting on FtsI in the periplasm to activate FtsWI to carry out sPG synthesis.
Keywords: FtsA; FtsQ; FtsW; cytokinesis; septal PG.
Conflict of interest statement
The authors declare no competing interest.
Figures









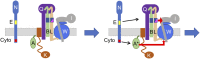
Similar articles
-
Essential Role for FtsL in Activation of Septal Peptidoglycan Synthesis.mBio. 2020 Dec 8;11(6):e03012-20. doi: 10.1128/mBio.03012-20. mBio. 2020. PMID: 33293384 Free PMC article.
-
A conserved subcomplex within the bacterial cytokinetic ring activates cell wall synthesis by the FtsW-FtsI synthase.Proc Natl Acad Sci U S A. 2020 Sep 22;117(38):23879-23885. doi: 10.1073/pnas.2004598117. Epub 2020 Sep 9. Proc Natl Acad Sci U S A. 2020. PMID: 32907942 Free PMC article.
-
FtsQ, FtsL and FtsI require FtsK, but not FtsN, for co-localization with FtsZ during Escherichia coli cell division.Mol Microbiol. 2001 Oct;42(2):395-413. doi: 10.1046/j.1365-2958.2001.02640.x. Mol Microbiol. 2001. PMID: 11703663
-
The cell cycle of Escherichia coli.Annu Rev Microbiol. 1993;47:199-230. doi: 10.1146/annurev.mi.47.100193.001215. Annu Rev Microbiol. 1993. PMID: 8257098 Review.
-
An Updated Model of the Divisome: Regulation of the Septal Peptidoglycan Synthesis Machinery by the Divisome.Int J Mol Sci. 2022 Mar 24;23(7):3537. doi: 10.3390/ijms23073537. Int J Mol Sci. 2022. PMID: 35408901 Free PMC article. Review.
Cited by
-
Structural insights into the activation of the divisome complex FtsWIQLB.Cell Discov. 2024 Jan 3;10(1):2. doi: 10.1038/s41421-023-00629-w. Cell Discov. 2024. PMID: 38172099 Free PMC article. No abstract available.
-
FtsN maintains active septal cell wall synthesis by forming a processive complex with the septum-specific peptidoglycan synthases in E. coli.Nat Commun. 2022 Sep 30;13(1):5751. doi: 10.1038/s41467-022-33404-8. Nat Commun. 2022. PMID: 36180460 Free PMC article.
-
Plasticity in the cell division processes of obligate intracellular bacteria.Front Cell Infect Microbiol. 2023 Oct 9;13:1205488. doi: 10.3389/fcimb.2023.1205488. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37876871 Free PMC article. Review.
-
A unique cell division protein critical for the assembly of the bacterial divisome.Elife. 2024 Oct 3;12:RP87922. doi: 10.7554/eLife.87922. Elife. 2024. PMID: 39361022 Free PMC article.
-
The coiled-coil domain of Escherichia coli FtsLB is a structurally detuned element critical for modulating its activation in bacterial cell division.J Biol Chem. 2022 Jan;298(1):101460. doi: 10.1016/j.jbc.2021.101460. Epub 2021 Dec 4. J Biol Chem. 2022. PMID: 34871549 Free PMC article.
References
-
- Goehring N. W., Beckwith J., Diverse paths to midcell: Assembly of the bacterial cell division machinery. Curr. Biol. 15, R514–R526 (2005). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

