Topoisomerase II is regulated by translationally controlled tumor protein for cell survival during organ growth in Drosophila
- PMID: 34453033
- PMCID: PMC8397738
- DOI: 10.1038/s41419-021-04091-y
Topoisomerase II is regulated by translationally controlled tumor protein for cell survival during organ growth in Drosophila
Abstract
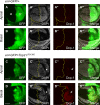
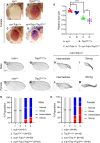
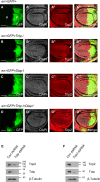
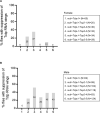
Regulation of cell survival is critical for organ development. Translationally controlled tumor protein (TCTP) is a conserved protein family implicated in the control of cell survival during normal development and tumorigenesis. Previously, we have identified a human Topoisomerase II (TOP2) as a TCTP partner, but its role in vivo has been unknown. To determine the significance of this interaction, we examined their roles in developing Drosophila organs. Top2 RNAi in the wing disc leads to tissue reduction and caspase activation, indicating the essential role of Top2 for cell survival. Top2 RNAi in the eye disc also causes loss of eye and head tissues. Tctp RNAi enhances the phenotypes of Top2 RNAi. The depletion of Tctp reduces Top2 levels in the wing disc and vice versa. Wing size is reduced by Top2 overexpression, implying that proper regulation of Top2 level is important for normal organ development. The wing phenotype of Tctp RNAi is partially suppressed by Top2 overexpression. This study suggests that mutual regulation of Tctp and Top2 protein levels is critical for cell survival during organ development.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Regulation of epithelial integrity and organ growth by Tctp and Coracle in Drosophila.PLoS Genet. 2020 Jun 19;16(6):e1008885. doi: 10.1371/journal.pgen.1008885. eCollection 2020 Jun. PLoS Genet. 2020. PMID: 32559217 Free PMC article.
-
Drosophila TCTP is essential for growth and proliferation through regulation of dRheb GTPase.Nature. 2007 Feb 15;445(7129):785-8. doi: 10.1038/nature05528. Nature. 2007. PMID: 17301792
-
The C-terminal 20 Amino Acids of Drosophila Topoisomerase 2 Are Required for Binding to a BRCA1 C Terminus (BRCT) Domain-containing Protein, Mus101, and Fidelity of DNA Segregation.J Biol Chem. 2016 Jun 17;291(25):13216-28. doi: 10.1074/jbc.M116.721357. Epub 2016 Apr 27. J Biol Chem. 2016. PMID: 27129233 Free PMC article.
-
Function of Translationally Controlled Tumor Protein in Organ Growth: Lessons from Drosophila Studies.Results Probl Cell Differ. 2017;64:173-191. doi: 10.1007/978-3-319-67591-6_8. Results Probl Cell Differ. 2017. PMID: 29149408 Review.
-
Organ Size Control: Lessons from Drosophila.Dev Cell. 2015 Aug 10;34(3):255-65. doi: 10.1016/j.devcel.2015.07.012. Dev Cell. 2015. PMID: 26267393 Free PMC article. Review.
Cited by
-
Tctp, a unique Ing5-binding partner, inhibits the chromatin binding of Enok in Drosophila.Proc Natl Acad Sci U S A. 2023 Apr 11;120(15):e2218361120. doi: 10.1073/pnas.2218361120. Epub 2023 Apr 4. Proc Natl Acad Sci U S A. 2023. PMID: 37014852 Free PMC article.
-
Genome-Wide CRISPR Screens Reveal ZATT as a Synthetic Lethal Target of TOP2-Poison Etoposide That Can Act in a TDP2-Independent Pathway.Int J Mol Sci. 2023 Mar 31;24(7):6545. doi: 10.3390/ijms24076545. Int J Mol Sci. 2023. PMID: 37047518 Free PMC article.
References
-
- Böhm H, Benndorf R, Gaestel M, Gross B, Nürnberg P, Kraft R, et al. The growth-related protein P23 of the Ehrlich ascites tumor: translational control, cloning and primary structure. Biochem Int. 1989;19:277–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NRF-2014K1A1A2042982/National Research Foundation of Korea (NRF)
- NRF-2017R1A2B3007516/National Research Foundation of Korea (NRF)
- NRF-2014K1A1A2042982/National Research Foundation of Korea (NRF)
- NRF-2017R1A2B3007516/National Research Foundation of Korea (NRF)
- NRF-2014K1A1A2042982/National Research Foundation of Korea (NRF)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

