Stimulus-dependent representational drift in primary visual cortex
- PMID: 34453051
- PMCID: PMC8397766
- DOI: 10.1038/s41467-021-25436-3
Stimulus-dependent representational drift in primary visual cortex
Erratum in
-
Author Correction: Stimulus-dependent representational drift in primary visual cortex.Nat Commun. 2021 Sep 10;12(1):5486. doi: 10.1038/s41467-021-25825-8. Nat Commun. 2021. PMID: 34508099 Free PMC article. No abstract available.
Abstract
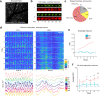
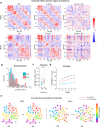
To produce consistent sensory perception, neurons must maintain stable representations of sensory input. However, neurons in many regions exhibit progressive drift across days. Longitudinal studies have found stable responses to artificial stimuli across sessions in visual areas, but it is unclear whether this stability extends to naturalistic stimuli. We performed chronic 2-photon imaging of mouse V1 populations to directly compare the representational stability of artificial versus naturalistic visual stimuli over weeks. Responses to gratings were highly stable across sessions. However, neural responses to naturalistic movies exhibited progressive representational drift across sessions. Differential drift was present across cortical layers, in inhibitory interneurons, and could not be explained by differential response strength or higher order stimulus statistics. However, representational drift was accompanied by similar differential changes in local population correlation structure. These results suggest representational stability in V1 is stimulus-dependent and may relate to differences in preexisting circuit architecture of co-tuned neurons.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

