Human osteoblast and fibroblast response to oral implant biomaterials functionalized with non-thermal oxygen plasma
- PMID: 34453071
- PMCID: PMC8397744
- DOI: 10.1038/s41598-021-96526-x
Human osteoblast and fibroblast response to oral implant biomaterials functionalized with non-thermal oxygen plasma
Abstract
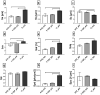
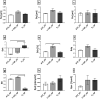
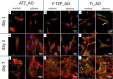
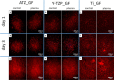
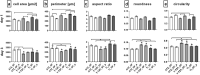
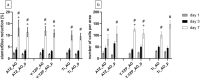
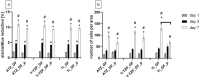
Plasma-treatment of oral implant biomaterials prior to clinical insertion is envisaged as a potential surface modification method for enhanced implant healing. To investigate a putative effect of plasma-functionalized implant biomaterials on oral tissue cells, this investigation examined the response of alveolar bone osteoblasts and gingival fibroblasts to clinically established zirconia- and titanium-based implant surfaces for bone and soft tissue integration. The biomaterials were either functionalized with oxygen-plasma in a plasma-cleaner or left untreated as controls, and were characterized in terms of topography and wettability. For the biological evaluation, the cell adhesion, morphogenesis, metabolic activity and proliferation were examined, since these parameters are closely interconnected during cell-biomaterial interaction. The results revealed that plasma-functionalization increased implant surface wettability. The magnitude of this effect thereby depended on surface topography parameters and initial wettability of the biomaterials. Concerning the cell response, plasma-functionalization of smooth surfaces affected initial fibroblast morphogenesis, whereas osteoblast morphology on rough surfaces was mainly influenced by topography. The plasma- and topography-induced differential cell morphologies were however not strong enough to trigger a change in proliferation behaviour. Hence, the results indicate that oxygen plasma-functionalization represents a possible cytocompatible implant surface modification method which can be applied for tailoring implant surface wettability.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


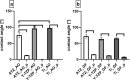



Similar articles
-
Air atmospheric-pressure plasma-jet treatment enhances the attachment of human gingival fibroblasts for early peri-implant soft tissue seals on titanium dental implant abutments.Acta Odontol Scand. 2015 Jan;73(1):67-75. doi: 10.3109/00016357.2014.954265. Epub 2014 Sep 3. Acta Odontol Scand. 2015. PMID: 25183251
-
Analysis of soft tissue integration-supportive cell functions in gingival fibroblasts cultured on 3D printed biomaterials for oral implant-supported prostheses.J Biomed Mater Res A. 2024 Sep;112(9):1376-1387. doi: 10.1002/jbm.a.37675. Epub 2024 Jan 22. J Biomed Mater Res A. 2024. PMID: 38251807
-
Surface properties correlated with the human gingival fibroblasts attachment on various materials for implant abutments: a multiple regression analysis.Acta Odontol Scand. 2015 Jan;73(1):38-47. doi: 10.3109/00016357.2014.949845. Epub 2014 Sep 3. Acta Odontol Scand. 2015. PMID: 25183254
-
Nano-scale modification of titanium implant surfaces to enhance osseointegration.Acta Biomater. 2019 Aug;94:112-131. doi: 10.1016/j.actbio.2019.05.045. Epub 2019 May 22. Acta Biomater. 2019. PMID: 31128320 Review.
-
Use of Plasma Technologies for Antibacterial Surface Properties of Metals.Molecules. 2021 Mar 5;26(5):1418. doi: 10.3390/molecules26051418. Molecules. 2021. PMID: 33808010 Free PMC article. Review.
Cited by
-
Laser Structured Dental Zirconium for Soft Tissue Cell Occupation-Importance of Wettability Modulation.Materials (Basel). 2022 Jan 19;15(3):732. doi: 10.3390/ma15030732. Materials (Basel). 2022. PMID: 35160678 Free PMC article.
-
Optimized Gingiva Cell Behavior on Dental Zirconia as a Result of Atmospheric Argon Plasma Activation.Materials (Basel). 2023 Jun 6;16(12):4203. doi: 10.3390/ma16124203. Materials (Basel). 2023. PMID: 37374388 Free PMC article.
-
Comparative Analysis of Osteoblastic Responses to Titanium and Alumina-Toughened Zirconia Implants: An In Vitro Study.Biomolecules. 2024 Jun 18;14(6):719. doi: 10.3390/biom14060719. Biomolecules. 2024. PMID: 38927122 Free PMC article.
-
Nonthermal Atmospheric Pressure Plasma Treatment of Endosteal Implants for Osseointegration and Antimicrobial Efficacy: A Comprehensive Review.Bioengineering (Basel). 2024 Mar 27;11(4):320. doi: 10.3390/bioengineering11040320. Bioengineering (Basel). 2024. PMID: 38671741 Free PMC article. Review.
-
Functionalization of gutta-percha surfaces with argon and oxygen plasma treatments to enhance adhesiveness.Sci Rep. 2023 Jul 29;13(1):12303. doi: 10.1038/s41598-023-37372-x. Sci Rep. 2023. PMID: 37516768 Free PMC article.
References
-
- Rompen E, Domken O, Degidi M, Pontes AEF, Piattelli A. The effect of material characteristics, of surface topography and of implant components and connections on soft tissue integration: A literature review. Clin. Oral Implants Res. 2006;17(Suppl 2):55–67. doi: 10.1111/j.1600-0501.2006.01367.x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

