Identification of potential autoantigens in anti-CCP-positive and anti-CCP-negative rheumatoid arthritis using citrulline-specific protein arrays
- PMID: 34453079
- PMCID: PMC8397748
- DOI: 10.1038/s41598-021-96675-z
Identification of potential autoantigens in anti-CCP-positive and anti-CCP-negative rheumatoid arthritis using citrulline-specific protein arrays
Abstract
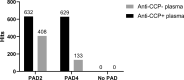
The presence or absence of autoantibodies against citrullinated proteins (ACPAs) distinguishes two main groups of rheumatoid arthritis (RA) patients with different etiologies, prognoses, disease severities, and, presumably, disease pathogenesis. The heterogeneous responses of RA patients to various biologics, even among ACPA-positive patients, emphasize the need for further stratification of the patients. We used high-density protein array technology for fingerprinting of ACPA reactivity. Identification of the proteome recognized by ACPAs may be a step to stratify RA patients according to immune reactivity. Pooled plasma samples from 10 anti-CCP-negative and 15 anti-CCP-positive RA patients were assessed for ACPA content using a modified protein microarray containing 1631 different natively folded proteins citrullinated in situ by protein arginine deiminases (PADs) 2 and PAD4. IgG antibodies from anti-CCP-positive RA plasma showed high-intensity binding to 87 proteins citrullinated by PAD2 and 99 proteins citrullinated by PAD4 without binding significantly to the corresponding native proteins. Curiously, the binding of IgG antibodies in anti-CCP-negative plasma was also enhanced by PAD2- and PAD4-mediated citrullination of 29 and 26 proteins, respectively. For only four proteins, significantly more ACPA binding occurred after citrullination with PAD2 compared to citrullination with PAD4, while the opposite was true for one protein. We demonstrate that PAD2 and PAD4 are equally efficient in generating citrullinated autoantigens recognized by ACPAs. Patterns of proteins recognized by ACPAs may serve as a future diagnostic tool for further subtyping of RA patients.
© 2021. The Author(s).
Conflict of interest statement
JMB is the Chief Scientific Officer of Sengenics Corporation who commercializes the Immunome arrays used in this study. TP, DD, MJ, CN, AS, and LS declare no competing interests.
Figures

References
-
- Schellekens GA, Visser H, de Jong BAW, Van Den Hoogen FHJ, Hazes JMW, Breedveld FC, et al. The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arthritis Rheum. 2000;43(1):155–163. doi: 10.1002/1529-0131(200001)43:1<155::AID-ANR20>3.0.CO;2-3. - DOI - PubMed
-
- Nielen MMJ, van Schaardenburg D, Reesink HW, van de Stadt RJ, van der Horst-Bruinsma IE, de Koning MHMT, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: A study of serial measurements in blood donors. Arthritis Rheum. 2004;50(2):380–386. doi: 10.1002/art.20018. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

