Effect of extracorporeal shock wave therapy on keratinocytes derived from human hypertrophic scars
- PMID: 34453089
- PMCID: PMC8397706
- DOI: 10.1038/s41598-021-96537-8
Effect of extracorporeal shock wave therapy on keratinocytes derived from human hypertrophic scars
Abstract
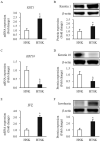
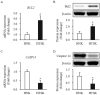
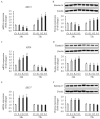
Hypertrophic scars represent a common complication in burn patients. In addition to cosmetic defects, they may cause serious sensory abnormalities such as pain and itching, severe dysfunction depending on the site, and emotional disorders such as anxiety and depression. The present study aimed to identify the molecular mechanisms underlying the use of extracorporeal shock wave therapy in keratinocytes. Keratinocytes derived from hypertrophic scar tissue were cultured and expression of proliferation markers (keratin 5 and 14), activation markers (keratin 6 and 17), differentiation markers (keratin 1, 10, and involucrin), apoptosis factors (Bax, Bcl2, and Caspase 14), and proliferation/differentiation regulators (p21 and p27) was investigated to compared with that of those in keratinocytes derived from normal skin tissue. Scar-derived keratinocytes were treated with extracorporeal shock waves under 1000 impulses at 0.1, 0.2, and 0.3 mJ/mm2. Shock waves altered the molecular pattern of proliferation, activation, differentiation, and apoptosis, as well as proliferation/ differentiation regulators, including Bax, Bcl2, ASK1, p21, p27, and Notch1. In summary, we show that extracorporeal shock wave therapy regulates the proliferation and differentiation of keratinocytes derived from hypertrophic scar to maintain normal epidermal integrity.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

