Omega-3 fatty acid blood levels are inversely associated with cardiometabolic risk factors in HFpEF patients: the Aldo-DHF randomized controlled trial
- PMID: 34453204
- PMCID: PMC8873063
- DOI: 10.1007/s00392-021-01925-9
Omega-3 fatty acid blood levels are inversely associated with cardiometabolic risk factors in HFpEF patients: the Aldo-DHF randomized controlled trial
Abstract
Objectives: To evaluate associations of omega-3 fatty acid (O3-FA) blood levels with cardiometabolic risk markers, functional capacity and cardiac function/morphology in patients with heart failure with preserved ejection fraction (HFpEF).
Background: O3-FA have been linked to reduced risk for HF and associated phenotypic traits in experimental/clinical studies.
Methods: This is a cross-sectional analysis of data from the Aldo-DHF-RCT. From 422 patients, the omega-3-index (O3I = EPA + DHA) was analyzed at baseline in n = 404 using the HS-Omega-3-Index® methodology. Patient characteristics were; 67 ± 8 years, 53% female, NYHA II/III (87/13%), ejection fraction ≥ 50%, E/e' 7.1 ± 1.5; median NT-proBNP 158 ng/L (IQR 82-298). Pearson's correlation coefficient and multiple linear regression analyses, using sex and age as covariates, were used to describe associations of the O3I with metabolic phenotype, functional capacity, echocardiographic markers for LVDF, and neurohumoral activation at baseline/12 months.
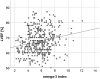
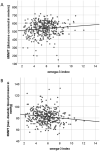
Results: The O3I was below (< 8%), within (8-11%), and higher (> 11%) than the target range in 374 (93%), 29 (7%), and 1 (0.2%) patients, respectively. Mean O3I was 5.7 ± 1.7%. The O3I was inversely associated with HbA1c (r = - 0.139, p = 0.006), triglycerides-to-HDL-C ratio (r = - 0.12, p = 0.017), triglycerides (r = - 0.117, p = 0.02), non-HDL-C (r = - 0.101, p = 0.044), body-mass-index (r = - 0.149, p = 0.003), waist circumference (r = - 0.121, p = 0.015), waist-to-height ratio (r = - 0.141, p = 0.005), and positively associated with submaximal aerobic capacity (r = 0.113, p = 0.023) and LVEF (r = 0.211, p < 0.001) at baseline. Higher O3I at baseline was predictive of submaximal aerobic capacity (β = 15.614, p < 0,001), maximal aerobic capacity (β = 0.399, p = 0.005) and LVEF (β = 0.698, p = 0.007) at 12 months.
Conclusions: Higher O3I was associated with a more favorable cardiometabolic risk profile and predictive of higher submaximal/maximal aerobic capacity and lower BMI/truncal adiposity in HFpEF patients. Omega-3 fatty acid blood levels are inversely associated with cardiometabolic risk factors in HFpEF patients. Higher O3I was associated with a more favorable cardiometabolic risk profile and aerobic capacity (left) but did not correlate with echocardiographic markers for left ventricular diastolic function or neurohumoral activation (right). An O3I-driven intervention trial might be warranted to answer the question whether O3-FA in therapeutic doses (with the target O3I 8-11%) impact on echocardiographic markers for left ventricular diastolic function and neurohumoral activation in patients with HFpEF. This figure contains modified images from Servier Medical Art ( https://smart.servier.com ) licensed by a Creative Commons Attribution 3.0 Unported License.
Keywords: Atherogenic dyslipidemia; Diastolic dysfunction; Docosahexaenoic acid; Eicosapentaenoic acid; Functional capacity; HFpEF; Heart failure; Metabolic phenotype; Omega-3 fatty acids; Omega-3 index.
© 2021. The Author(s).
Conflict of interest statement
KL, JS, EL, BL, BH, AK, MH, RW, AD, FE declare that they have no conflict of interest to disclose with respect to this manuscript.
Figures


References
-
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. - PubMed
-
- Pieske B, Tschöpe C, de Boer RA, et al. How to diagnose heart failure with preserved ejection fraction: the HFA–PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC) Eur Heart J. 2019;40:3297–3317. - PubMed
-
- Packer M. Epicardial adipose tissue may mediate deleterious effects of obesity and inflammation on the myocardium. J Am Coll Cardiol. 2018;71:2360–2372. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

