Efficacy and Safety of Favipiravir in Moderate COVID-19 Pneumonia Patients without Oxygen Therapy: A Randomized, Phase III Clinical Trial
- PMID: 34453234
- PMCID: PMC8396144
- DOI: 10.1007/s40121-021-00517-4
Efficacy and Safety of Favipiravir in Moderate COVID-19 Pneumonia Patients without Oxygen Therapy: A Randomized, Phase III Clinical Trial
Abstract
Introduction: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the cause of coronavirus disease 2019 (COVID-19), is an enveloped, single-stranded RNA virus. Favipiravir is an orally administrable antiviral drug whose mechanism of action is to selectively inhibit RNA-dependent RNA polymerase. A preliminary trial in COVID-19 patients reported significant improvements across a multitude of clinical parameters, but these findings have not been confirmed in an adequate well-controlled trial. We conducted a randomized, single-blind, placebo-controlled Phase III trial assessing the efficacy and safety of favipiravir in patients with moderate pneumonia not requiring oxygen therapy.
Methods: COVID-19 patients with moderate pneumonia (SpO2 ≥ 94%) within 10 days of onset of fever (temperature ≥ 37.5 °C) were assigned to receive either placebo or favipiravir (1800 mg twice a day on Day 1, followed by 800 mg twice a day for up to 13 days) in a ratio of 1:2. An adaptive design was used to re-estimate the sample size. The primary endpoint was a composite outcome defined as the time to improvement in temperature, oxygen saturation levels (SpO2), and findings on chest imaging, and recovery to SARS-CoV-2-negative. This endpoint was re-examined by the Central Committee under blinded conditions.
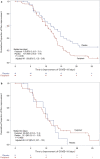
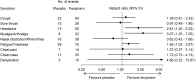
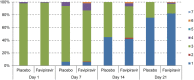
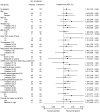
Results: A total of 156 patients were randomized. The median time of the primary endpoint was 11.9 days in the favipiravir group and 14.7 days in the placebo group, with a significant difference (p = 0.0136). Favipiravir-treated patients with known risk factors such as obesity or coexisting conditions provided better effects. Furthermore, patients with early-onset in the favipiravir group showed higher odds ratio. No deaths were documented. Although adverse events in the favipiravir group were predominantly transient, the incidence was significantly higher.
Conclusions: The results suggested favipiravir may be one of options for moderate COVID-19 pneumonia treatment. However, the risk of adverse events, including hyperuricemia, should be carefully considered.
Trial registration: Clinicaltrials.jp number: JapicCTI-205238.
Keywords: COVID-19; Favipiravir; Moderate pneumonia not requiring oxygen therapy; Oral antiviral agent; Phase III clinical trial; SARS-CoV-2; Treatment efficacy.
© 2021. The Author(s).
Figures



References
-
- Coronavirus disease (COVID-19) Weekly Epidemiological Update and Weekly Operational Upd. Geneva: World Health Organization. 2021. https://www.who.int/publications/m/item/weekly-operational-update-on-cov.... Accessed 10 May 2021.
LinkOut - more resources
Full Text Sources
Miscellaneous

