Quantitative Systems Pharmacology Modeling of Avadomide-Induced Neutropenia Enables Virtual Clinical Dose and Schedule Finding Studies
- PMID: 34453265
- PMCID: PMC8397660
- DOI: 10.1208/s12248-021-00623-8
Quantitative Systems Pharmacology Modeling of Avadomide-Induced Neutropenia Enables Virtual Clinical Dose and Schedule Finding Studies
Erratum in
-
Correction to: Quantitative Systems Pharmacology Modeling of Avadomide-Induced Neutropenia Enables Virtual Clinical Dose and Schedule Finding Studies.AAPS J. 2022 Jan 14;24(1):29. doi: 10.1208/s12248-021-00673-y. AAPS J. 2022. PMID: 35038051 Free PMC article. No abstract available.
Abstract
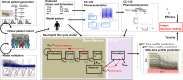
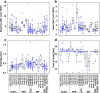
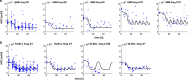
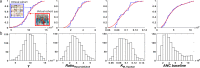
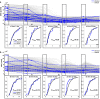
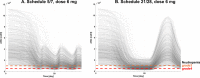
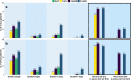
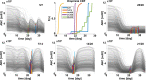
Avadomide is a cereblon E3 ligase modulator and a potent antitumor and immunomodulatory agent. Avadomide trials are challenged by neutropenia as a major adverse event and a dose-limiting toxicity. Intermittent dosing schedules supported by preclinical data provide a strategy to reduce frequency and severity of neutropenia; however, the identification of optimal dosing schedules remains a clinical challenge. Quantitative systems pharmacology (QSP) modeling offers opportunities for virtual screening of efficacy and toxicity levels produced by alternative dose and schedule regimens, thereby supporting decision-making in translational drug development. We formulated a QSP model to capture the mechanism of avadomide-induced neutropenia, which involves cereblon-mediated degradation of transcription factor Ikaros, resulting in a maturation block of the neutrophil lineage. The neutropenia model was integrated with avadomide-specific pharmacokinetic and pharmacodynamic models to capture dose-dependent effects. Additionally, we generated a disease-specific virtual patient population to represent the variability in patient characteristics and response to treatment observed for a diffuse large B-cell lymphoma trial cohort. Model utility was demonstrated by simulating the avadomide effect in the virtual population for various dosing schedules and determining the incidence of high-grade neutropenia, its duration, and the probability of recovery to low-grade neutropenia.
Keywords: CELMoD; QSP; avadomide; neutropenia; virtual patient.
© 2021. The Author(s).
Conflict of interest statement
RAA, MP, SC, DWP, SK, MM, MWBT, RL, and AVR declare employment at Bristol Myers Squibb. MP, SC, DWP, SK, MM, MWBT, RL, CCS, AVR declare equity ownership in Bristol Myers Squibb.
Figures
Similar articles
-
A First-in-Human Study of Novel Cereblon Modulator Avadomide (CC-122) in Advanced Malignancies.Clin Cancer Res. 2019 Jan 1;25(1):90-98. doi: 10.1158/1078-0432.CCR-18-1203. Epub 2018 Sep 10. Clin Cancer Res. 2019. PMID: 30201761 Clinical Trial.
-
Avadomide monotherapy in relapsed/refractory DLBCL: safety, efficacy, and a predictive gene classifier.Blood. 2020 Mar 26;135(13):996-1007. doi: 10.1182/blood.2019002395. Blood. 2020. PMID: 31977002 Free PMC article.
-
Avadomide plus obinutuzumab in patients with relapsed or refractory B-cell non-Hodgkin lymphoma (CC-122-NHL-001): a multicentre, dose escalation and expansion phase 1 study.Lancet Haematol. 2020 Sep;7(9):e649-e659. doi: 10.1016/S2352-3026(20)30208-8. Epub 2020 Aug 3. Lancet Haematol. 2020. PMID: 32758434 Clinical Trial.
-
Pharmacokinetics and pharmacodynamics of pegfilgrastim.Clin Pharmacokinet. 2011 May;50(5):295-306. doi: 10.2165/11586040-000000000-00000. Clin Pharmacokinet. 2011. PMID: 21456630 Review.
-
[Prevention and treatment of febrile neutropenia].Tumori. 1997;83(2 Suppl):S15-9. Tumori. 1997. PMID: 9235724 Review. Italian.
Cited by
-
Applications of Quantitative Systems Pharmacology (QSP) in Drug Development for NAFLD and NASH and Its Regulatory Application.Pharm Res. 2022 Aug;39(8):1789-1802. doi: 10.1007/s11095-022-03295-x. Epub 2022 May 24. Pharm Res. 2022. PMID: 35610402 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

