SARS-CoV-2 persistence in immunocompromised children
- PMID: 34453477
- PMCID: PMC8661864
- DOI: 10.1002/pbc.29277
SARS-CoV-2 persistence in immunocompromised children
Abstract
Objectives: We evaluated the length of time immunocompromised children (ICC) remain positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), identified factors associated with viral persistence, and determined cycle threshold (CT ) values of children with viral persistence as a surrogate of viral load.
Methods: We conducted a retrospective cohort study of ICC at a pediatric hospital from March 2020 to March 2021. Immunocompromised status was defined as primary, secondary, or acquired due to medical comorbidities/immunosuppressive treatment. The primary outcome was time to first of two consecutive negative SARS-CoV-2 polymerase chain reaction (PCR) tests at least 24 hours apart. Testing of sequential clinical specimens from the same subject was conducted using the Centers for Disease Control (CDC) 2019-nCoV real-time reverse transcriptase (RT)-PCR Diagnostic Panel assay. Descriptive statistics, Kaplan-Meier curve median event times and log-rank tests were used to compare outcomes between groups.
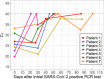
Results: Ninety-one children met inclusion criteria. Median age was 15.5 years (interquartile range [IQR] 8-18), 64% were male, 58% were White, and 43% were Hispanic/Latinx. Most (67%) were tested in outpatient settings and 58% were asymptomatic. The median time to two negative tests was 42 days (IQR 25.0-55.0), with no differences in median time by illness presentation or level of immunosuppression. Seven children had more than one sample available for repeat testing, and five of seven (71%) children had initial CT values of <30 (moderate to high viral load); four children had CT values of <30, 3-4 weeks later, suggesting persistent moderate to high viral loads.
Conclusions: Most ICC with SARS-CoV-2 infection had mild disease, with prolonged viral persistence >6 weeks and moderate to high viral load.
Keywords: COVID-19; SARS CoV-2; immunocompromised; pediatrics; shedding; viral persistence.
© 2021 Wiley Periodicals LLC.
Conflict of interest statement
Suchitra Rao and Samuel R. Dominguez received research support from BioFire Diagnostics. The remaining authors have no conflict of interest to disclose.
Figures


Comment in
-
Posterior reversible encephalopathy syndrome and necrotizing enterocolitis in a pediatric patient with medulloblastoma and COVID-19 infection.Pediatr Blood Cancer. 2023 Jan;70(1):e29868. doi: 10.1002/pbc.29868. Epub 2022 Jul 16. Pediatr Blood Cancer. 2023. PMID: 35841310 Free PMC article. No abstract available.
References
-
- Sethuraman N, Jeremiah SS, Ryo A. Interpreting diagnostic tests for SARS‐CoV‐2. JAMA. 2020;323(22):2249‐2251. - PubMed
-
- Kaltsas A, Sepkowitz K. Community acquired respiratory and gastrointestinal viral infections: challenges in the immunocompromised host. Curr Opin Infect Dis. 2012;25(4):423‐430. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

