Genetic reduction of mTOR extends lifespan in a mouse model of Hutchinson-Gilford Progeria syndrome
- PMID: 34453483
- PMCID: PMC8441492
- DOI: 10.1111/acel.13457
Genetic reduction of mTOR extends lifespan in a mouse model of Hutchinson-Gilford Progeria syndrome
Abstract
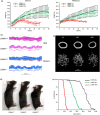
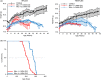
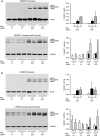
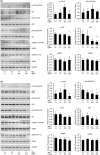
Hutchinson-Gilford progeria syndrome (HGPS) is a rare accelerated aging disorder most notably characterized by cardiovascular disease and premature death from myocardial infarction or stroke. The majority of cases are caused by a de novo single nucleotide mutation in the LMNA gene that activates a cryptic splice donor site, resulting in production of a toxic form of lamin A with a 50 amino acid internal deletion, termed progerin. We previously reported the generation of a transgenic murine model of progeria carrying a human BAC harboring the common mutation, G608G, which in the single-copy state develops features of HGPS that are limited to the vascular system. Here, we report the phenotype of mice bred to carry two copies of the BAC, which more completely recapitulate the phenotypic features of HGPS in skin, adipose, skeletal, and vascular tissues. We further show that genetic reduction of the mechanistic target of rapamycin (mTOR) significantly extends lifespan in these mice, providing a rationale for pharmacologic inhibition of the mTOR pathway in the treatment of HGPS.
Keywords: S6 Kinase; lamin A/C; laminopathies; mTOR; progeria.
© 2021 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Cao, K., Graziotto, J. J., Blair, C. D., Mazzulli, J. R., Erdos, M. R., Krainc, D., & Collins, F. S. (2011). Rapamycin reverses cellular phenotypes and enhances mutant protein clearance in Hutchinson‐Gilford progeria syndrome cells. Science Translational Medicine, 3(89), 89ra58. 10.1126/scitranslmed.3002346 - DOI - PubMed
-
- Capell, B. C., Olive, M., Erdos, M. R., Cao, K., Faddah, D. A., Tavarez, U. L., Conneely, K. N., Qu, X., San, H., Ganesh, S. K., Chen, X., Avallone, H., Kolodgie, F. D., Virmani, R., Nabel, E. G., & Collins, F. S. (2008). A farnesyltransferase inhibitor prevents both the onset and late progression of cardiovascular disease in a progeria mouse model. Proceedings of the National Academy of Sciences, 105(41), 15902–15907. 10.1073/pnas.0807840105 - DOI - PMC - PubMed
-
- Cenni, V., Capanni, C., Columbaro, M., Ortolani, M., D'Apice, M. R., Novelli, G., Fini, M., Marmiroli, S., Scarano, E., Maraldi, N. M., Squarzoni, S., Prencipe, S., & Lattanzi, G. (2011). Autophagic degradation of farnesylated prelamin A as a therapeutic approach to lamin‐linked progeria. European Journal of Histochemistry, 55(4), e36. 10.4081/ejh.2011.e36 - DOI - PMC - PubMed
-
- Cubria, M. B., Suarez, S., Masoudi, A., Oftadeh, R., Kamalapathy, P., DuBose, A., Erdos, M. R., Cabral, W. A., Karim, L., Collins, F. S., Snyder, B. D., & Nazarian, A. (2020). Evaluation of musculoskeletal phenotype of the G608G progeria mouse model with lonafarnib, pravastatin, and zoledronic acid as treatment groups. Proceedings of the National Academy of Sciences, 117(22), 12029–12040. 10.1073/pnas.1906713117 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

