Upper limb disease evolution in exon 53 skipping eligible patients with Duchenne muscular dystrophy
- PMID: 34453498
- PMCID: PMC8528463
- DOI: 10.1002/acn3.51417
Upper limb disease evolution in exon 53 skipping eligible patients with Duchenne muscular dystrophy
Abstract
Objective: To understand the natural disease upper limb progression over 3 years of ambulatory and non-ambulatory patients with Duchenne muscular dystrophy (DMD) using functional assessments and quantitative magnetic resonance imaging (MRI) and to exploratively identify prognostic factors.
Methods: Forty boys with DMD (22 non-ambulatory and 18 ambulatory) with deletions in dystrophin that make them eligible for exon 53-skipping therapy were included. Clinical assessments, including Brooke score, motor function measure (MFM), hand grip and key pinch strength, and upper limb distal coordination and endurance (MoviPlate), were performed every 6 months and quantitative MRI of fat fraction (FF) and lean muscle cross sectional area (flexor and extensor muscles) were performed yearly.
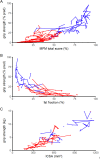
Results: In the whole population, there were strong nonlinear correlations between outcome measures. In non-ambulatory patients, annual changes over the course of 3 years were detected with high sensitivity standard response mean (|SRM| ≥0.8) for quantitative MRI-based FF, hand grip and key pinch, and MFM. Boys who presented with a FF<20% and a grip strength >27% were able to bring a glass to their mouth and retained this ability in the following 3 years. Ambulatory patients with grip strength >35% of predicted value and FF <10% retained ambulation 3 years later.
Interpretation: We demonstrate that continuous decline in upper limb strength, function, and MRI measured muscle structure can be reliably measured in ambulatory and non-ambulatory boys with DMD with high SRM and strong correlations between outcomes. Our results suggest that a combination of grip strength and FF can be used to predict important motor milestones.
© 2021 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
CL has no direct disclosures to declare; she is an MFM, ActiMyo, and ATOM trainer. HR, AMS, TG, MA, VC, VD, IL, and JLL report no disclosures relevant to the manuscript. EG is president of BIOSSEC and was hired by Généthon for the statistical analyses. FM has no direct disclosures to declare; he consults for Pfizer, Sarepta, Santhera, and Dyne Therapeutics. JYH is a coinventor of the MyoGrip, MyoPinch, and MoviPlate. PGC receives support from the European Community and the
Figures
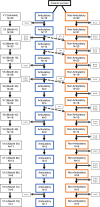
 ) loss of ambulation.
) loss of ambulation.

Similar articles
-
Muscle-MRI and Functional Levels for the Evaluation of Upper Limbs in Duchenne Muscular Dystrophy: A Critical Review of the Literature.Medicina (Kaunas). 2022 Mar 17;58(3):440. doi: 10.3390/medicina58030440. Medicina (Kaunas). 2022. PMID: 35334617 Free PMC article. Review.
-
Upper Limb Evaluation in Duchenne Muscular Dystrophy: Fat-Water Quantification by MRI, Muscle Force and Function Define Endpoints for Clinical Trials.PLoS One. 2016 Sep 20;11(9):e0162542. doi: 10.1371/journal.pone.0162542. eCollection 2016. PLoS One. 2016. PMID: 27649492 Free PMC article.
-
Respiratory and upper limb function as outcome measures in ambulant and non-ambulant subjects with Duchenne muscular dystrophy: A prospective multicentre study.Neuromuscul Disord. 2019 Apr;29(4):261-268. doi: 10.1016/j.nmd.2019.02.002. Epub 2019 Feb 19. Neuromuscul Disord. 2019. PMID: 30852071
-
The association of hand grip strength with functional measures in non-ambulatory children with Duchenne muscular dystrophy.Arq Neuropsiquiatr. 2019 Nov;77(11):792-796. doi: 10.1590/0004-282X20190161. Arq Neuropsiquiatr. 2019. PMID: 31826135
-
Contributions of Japanese patients to development of antisense therapy for DMD.Brain Dev. 2016 Jan;38(1):4-9. doi: 10.1016/j.braindev.2015.05.014. Epub 2015 Jun 18. Brain Dev. 2016. PMID: 26094594 Review.
Cited by
-
Upper Limb Changes in DMD Patients Amenable to Skipping Exons 44, 45, 51 and 53: A 24-Month Study.Children (Basel). 2023 Apr 19;10(4):746. doi: 10.3390/children10040746. Children (Basel). 2023. PMID: 37189996 Free PMC article.
-
MRI Assessment of Motor Capabilities in Patients with Duchenne Muscular Dystrophy According to the Motor Function Measure Scale.Tomography. 2022 Apr 1;8(2):948-960. doi: 10.3390/tomography8020076. Tomography. 2022. PMID: 35448710 Free PMC article.
-
Muscle-MRI and Functional Levels for the Evaluation of Upper Limbs in Duchenne Muscular Dystrophy: A Critical Review of the Literature.Medicina (Kaunas). 2022 Mar 17;58(3):440. doi: 10.3390/medicina58030440. Medicina (Kaunas). 2022. PMID: 35334617 Free PMC article. Review.
-
Draft Guidance for Industry Duchenne Muscular Dystrophy, Becker Muscular Dystrophy, and Related Dystrophinopathies - Developing Potential Treatments for the Entire Spectrum of Disease.J Neuromuscul Dis. 2024;11(2):499-523. doi: 10.3233/JND-230219. J Neuromuscul Dis. 2024. PMID: 38363616 Free PMC article.
-
Clinical importance of changes in magnetic resonance biomarkers for Duchenne muscular dystrophy.Ann Clin Transl Neurol. 2024 Jan;11(1):67-78. doi: 10.1002/acn3.51933. Epub 2023 Nov 6. Ann Clin Transl Neurol. 2024. PMID: 37932907 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

