Rapid induction of antigen-specific CD4+ T cells is associated with coordinated humoral and cellular immunity to SARS-CoV-2 mRNA vaccination
- PMID: 34453880
- PMCID: PMC8361141
- DOI: 10.1016/j.immuni.2021.08.001
Rapid induction of antigen-specific CD4+ T cells is associated with coordinated humoral and cellular immunity to SARS-CoV-2 mRNA vaccination
Abstract
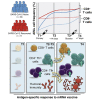
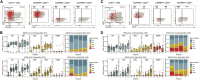
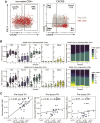
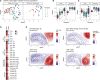
SARS-CoV-2 mRNA vaccines have shown remarkable clinical efficacy, but questions remain about the nature and kinetics of T cell priming. We performed longitudinal antigen-specific T cell analyses on healthy SARS-CoV-2-naive and recovered individuals prior to and following mRNA prime and boost vaccination. Vaccination induced rapid antigen-specific CD4+ T cell responses in naive subjects after the first dose, whereas CD8+ T cell responses developed gradually and were variable in magnitude. Vaccine-induced Th1 and Tfh cell responses following the first dose correlated with post-boost CD8+ T cells and neutralizing antibodies, respectively. Integrated analysis revealed coordinated immune responses with distinct trajectories in SARS-CoV-2-naive and recovered individuals. Last, whereas booster vaccination improved T cell responses in SARS-CoV-2-naive subjects, the second dose had little effect in SARS-CoV-2-recovered individuals. These findings highlight the role of rapidly primed CD4+ T cells in coordinating responses to the second vaccine dose in SARS-CoV-2-naive individuals.
Keywords: AIM; SARS-CoV-2; T cells; T follicular helper; Tfh; Th1; activation induced markers; immunological memory; mRNA vaccine.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests S.E.H. has received consultancy fees from Sanofi Pasteur, Lumen, Novavax, and Merk for work unrelated to this study. E.J.W. is a consultant or an adviser for Merck, Elstar, Janssen, Related Sciences, Synthekine, and Surface Oncology. E.J.W. is a founder of Surface Oncology and Arsenal Biosciences. E.J.W. is an inventor on a patent (U.S. Patent No. 10,370,446) submitted by Emory University that covers the use of PD-1 blockade to treat infections and cancer. A.S. is a consultant for Gritstone, Flow Pharma, CellCarta, Arcturus, Oxfordimmunotech, and Avalia. La Jolla Institute for Immunology has filed for patent protection for various aspects of T cell epitope and vaccine design work.
Figures

References
-
- Abu Jabal K., Ben-Amram H., Beiruti K., Batheesh Y., Sussan C., Zarka S., Edelstein M. Impact of age, ethnicity, sex and prior infection status on immunogenicity following a single dose of the BNT162b2 mRNA COVID-19 vaccine: real-world evidence from healthcare workers, Israel, December 2020 to January 2021. Euro Surveil. 2021;26:2100096. - PMC - PubMed
-
- Acosta-Rodriguez E.V., Rivino L., Geginat J., Jarrossay D., Gattorno M., Lanzavecchia A., Sallusto F., Napolitani G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat. Immunol. 2007;8:639–646. - PubMed
-
- Angyal A., Longet S., Moore S., Payne R.P., Harding A., Tipton T., Rongkard P., Ali M., Hering L.M., Meardon N., et al. T-cell and antibody responses to first BNT162b2 vaccine dose in previously SARS-CoV-2-infected and infection-naive UK healthcare workers: a multicentre, prospective, observational cohort study. 2021. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3820576 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

