Repeated strand invasion and extensive branch migration are hallmarks of meiotic recombination
- PMID: 34453891
- PMCID: PMC8541907
- DOI: 10.1016/j.molcel.2021.08.003
Repeated strand invasion and extensive branch migration are hallmarks of meiotic recombination
Abstract
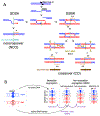
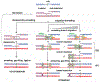
Currently favored models for meiotic recombination posit that both noncrossover and crossover recombination are initiated by DNA double-strand breaks but form by different mechanisms: noncrossovers by synthesis-dependent strand annealing and crossovers by formation and resolution of double Holliday junctions centered around the break. This dual mechanism hypothesis predicts different hybrid DNA patterns in noncrossover and crossover recombinants. We show that these predictions are not upheld, by mapping with unprecedented resolution parental strand contributions to recombinants at a model locus. Instead, break repair in both noncrossovers and crossovers involves synthesis-dependent strand annealing, often with multiple rounds of strand invasion. Crossover-specific double Holliday junction formation occurs via processes involving branch migration as an integral feature, one that can be separated from repair of the break itself. These findings reveal meiotic recombination to be a highly dynamic process and prompt a new view of the relationship between crossover and noncrossover recombination.
Keywords: Saccharomyces; branch migration; crossover; double Holliday junction; end extension; gene conversion; homologous recombination; meiosis; noncrossover; strand invasion.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

