Rotator cuff repair using a bioresorbable nanofiber interposition scaffold: a biomechanical and histologic analysis in sheep
- PMID: 34454041
- PMCID: PMC9364572
- DOI: 10.1016/j.jse.2021.07.018
Rotator cuff repair using a bioresorbable nanofiber interposition scaffold: a biomechanical and histologic analysis in sheep
Abstract
Background: The purpose of this study was to evaluate the mechanical, structural, and histologic quality of rotator cuff repairs augmented with an interposition electrospun nanofiber scaffold composed of polyglycolic acid (PGA) and poly-L-lactide-co-ε-caprolactone (PLCL) in an acute sheep model.
Methods: Forty acute infraspinatus tendon detachment and repair procedures were performed in a sheep infraspinatus model using a double-row transosseous-equivalent anchor technique either with an interposition nanofiber scaffold composed of polyglycolic acid-poly-L-lactide-co-ε-caprolactone or with no scaffold. Animals were euthanized at the 6-week (20 samples) and 12-week (20 samples) postoperative time points to assess the biomechanical and histologic properties of the repairs and to compare differences within each group.
Results: Within the scaffold-treated group, there was a significant increase in ultimate failure force (in newtons) from 6 to 12 weeks (P < .01), a significant increase in ultimate failure load from 6 to 12 weeks (P < .01), and a significant increase in ultimate failure stress (in megapascals) from 6 to 12 weeks (P < .01). At 6 weeks, the tendon-bone attachment was most consistent with an "indirect" type of insertion, whereas at 12 weeks, a visible difference in the progression and re-formation of the enthesis was observed. Compared with controls, animals in the scaffold-treated group displayed an insertion of the fibrous tendon with the humeral footprint that was beginning to be organized in a manner similar to the "native" direct/fibrocartilaginous insertion of the ovine infraspinatus tendon. In the majority of these animals treated with the scaffold, prominent perforating collagen fibers, similar to Sharpey fibers, were present and extending through a region of calcified fibrocartilage and attaching to the humeral footprint. No surgical complications occurred in any of the 40 sheep, including delayed wound healing or infection.
Conclusions: In a sheep acute rotator cuff repair model, securing a nanofiber scaffold between the tendon and the bone using a double-row transosseous-equivalent anchor fixation technique resulted in greater failure strength. Additionally, at the enthesis, Sharpey fiber-like attachments (ie, collagen fibers extending from the tendon into the calcified fibrocartilage of the humerus) were observed, which were not seen in the control group.
Keywords: Rotator cuff repair (RCR); acute model; nanofiber; ovine model; scaffold; tendon.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
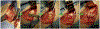



 ), in addition to the tendon (*) and residual scaffold (#) at the 6-week time point.
), in addition to the tendon (*) and residual scaffold (#) at the 6-week time point.
 ). When present, these fibers were characterized as broad, distinctive bundles of dense collagen that originated from the tendon fibrous connective tissue, extended through fibrocartilage or hyaline-like cartilage, and attached to the underlying humeral bone along the tendon-bone interface region of interest (nanofiber treatment, 12 weeks, magnification ×10).
). When present, these fibers were characterized as broad, distinctive bundles of dense collagen that originated from the tendon fibrous connective tissue, extended through fibrocartilage or hyaline-like cartilage, and attached to the underlying humeral bone along the tendon-bone interface region of interest (nanofiber treatment, 12 weeks, magnification ×10).References
-
- Agudelo-Garcia PA, De Jesus JK, Williams SP, Nowicki MO, Chiocca EA, Liyanarachchi S, et al. Glioma cell migration on three-dimensional nanofiber scaffolds is regulated by substrate topography and abolished by inhibition of STAT3 signaling. Neoplasia 2011;13:831–40. 10.1593/neo.11612 - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

