Hydroxychloroquine plus azithromycin early treatment of mild COVID-19 in an outpatient setting: a randomized, double-blinded, placebo-controlled clinical trial evaluating viral clearance
- PMID: 34454044
- PMCID: PMC8386096
- DOI: 10.1016/j.ijantimicag.2021.106428
Hydroxychloroquine plus azithromycin early treatment of mild COVID-19 in an outpatient setting: a randomized, double-blinded, placebo-controlled clinical trial evaluating viral clearance
Abstract
Background: Hydroxychloroquine has shown potential to block viral replication of SARS-CoV-2 in some in vitro studies. This randomised, double-blinded, placebo controlled clinical trial evaluated the efficacy of hydroxychloroquine plus azithromycin (HCQ/AZT) in reducing viral loads in patients with early and mild SARS-CoV-2 infection.
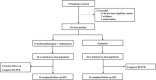
Methods: A single-centre randomised placebo-controlled clinical trial was conducted with outpatients with early and mild SARS-CoV-2 infection. Inclusion criteria were: patients aged 18-65 years with symptoms suggestive of COVID-19 for < 5 days, no significant comorbidities, and positive nasopharyngeal/oropharyngeal swab screening tests (POCT-PCR). Randomised patients received either hydroxychloroquine for 7 days plus azithromycin for 5 days or placebo. The primary endpoint was viral clearance within a 9-day period. Secondary endpoints included viral load reduction, clinical evolution, hospitalization rates, chest computed tomography evolution, and adverse effects.
Results: From 107 potential trial participants, 84 were enrolled following predetermined criteria. Statistical analyses were performed on an intention-to-treat (N = 84) and per-protocol (PP) basis (N = 70). On the PP analysis, the treatment (N = 36) and placebo (N = 34) groups displayed similar demographic characteristics. At 95% CI, no statistically significant between-group differences were found in viral clearance rates within 9 days following enrolment (P = 0.26).
Conclusions: This randomised, double-blinded, placebo-controlled clinical trial evaluating outpatients with early and mild COVID-19 showed that viral clearance rates within a 9-day period from enrolment did not change with HCQ/AZT treatment compared with placebo, although no major cardiovascular events were observed in participants without comorbidities. Secondary outcomes were also not significantly improved with HCQ/AZT treatment compared with placebo. These findings do not support use of HCQ/AZT in this setting.
Keywords: Azithromycin; COVID-19; Hydroxychloroquine; RCT; SARS-CoV-2.
Copyright © 2021 Elsevier Ltd and International Society of Antimicrobial Chemotherapy. All rights reserved.
References
-
- Organization GWH. WHO coronavirus disease (COVID-19) dashboard. Available at: https://covid19.who.int/[accessed 07 June 2021 ].
-
- Dong L, Hu S, Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov Ther. 2020;14(1):58–60. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous