HSymM-guided engineering of the immunodominant p53 transactivation domain putative peptide antigen for improved binding to its anti-p53 monoclonal antibody
- PMID: 34454062
- PMCID: PMC8526406
- DOI: 10.1016/j.bmcl.2021.128341
HSymM-guided engineering of the immunodominant p53 transactivation domain putative peptide antigen for improved binding to its anti-p53 monoclonal antibody
Abstract
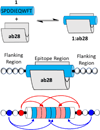
A novel engineering strategy to improve autoantibody detection with peptide fragments derived from the parent antigen is presented. The model system studied was the binding of the putative p53 TAD peptide antigen (residues 46-55) to its cognate anti-p53 antibody, ab28. Each engineered peptide contained the full decapeptide epitope and differed only in the flanking regions. Since minimal structural information was available to guide the design, a simple epitope:paratope binding model was applied. The Hidden Symmetry Model, which we recently reported, was used to guide peptide design and estimate per-residue contributions to interaction free energy as a function of added C- and N-terminal flanking peptides. Twenty-four peptide constructs were designed, synthesized, and assessed for binding affinity to ab28 by surface plasmon resonance, and a subset of these peptides were evaluated in a simulated immunoassay for limit of detection. Many peptides exhibited over 200-fold enhancements in binding affinity and improved limits of detection. The epitope was reevaluated and is proposed to be the undecapeptide corresponding to residues 45-55. HSymM calculated binding free energy and experimental data were found to be in good agreement (R2 > 0.75).
Keywords: Antibody; Autoantibodies; Coarse grained models; Epitope; Hidden symmetry model; Immunoassays; Paratope; Peptide engineering; Peptides; p53.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Figures
References
-
- Pedersen JW; Gentry-Maharaj A; Fourkala EO; Dawnay A; Burnell M; Zaikin A; Pedersen AE; Jacobs I; Menon U; Wandall HH, Early detection of cancer in the general population: a blinded case-control study of p53 autoantibodies in colorectal cancer. British journal of cancer 2013, 108 (1), 107–14. - PMC - PubMed
-
- Macdonald IK; Parsy-Kowalska CB; Chapman CJ, Autoantibodies: Opportunities for Early Cancer Detection. Trends in Cancer 2017, 3 (3), 198–213. - PubMed
-
- Qin J; Zeng N; Yang T; Wan C; Chen L; Shen Y; Wen F, Diagnostic Value of Autoantibodies in Lung Cancer: a Systematic Review and Meta-Analysis. Cellular Physiology and Biochemistry 2018, 51 (6), 2631–2646. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

