Efficacy of PD-1/PD-L1 inhibitors in ovarian cancer: a single-arm meta-analysis
- PMID: 34454562
- PMCID: PMC8403414
- DOI: 10.1186/s13048-021-00862-5
Efficacy of PD-1/PD-L1 inhibitors in ovarian cancer: a single-arm meta-analysis
Abstract
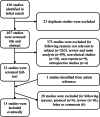
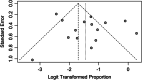
Several studies have evaluated the efficacy of PD-1/PD-L1 inhibitors in ovarian cancer; however, the response rate varies. This study aims to explore the efficacy of anti-PD-1/PD-L1 therapy in ovarian cancer. A quantitative meta-analysis was performed through a systematic search in PubMed, Web of Science, and the Cochrane Library. The pooled ORR was calculated and compared. Fifteen trials were included in this meta-analysis. Our analyses showed that the pooled ORR of all included studies was 19% (95% CI: 13%, 27%). Single PD-1/PD-L1 inhibitors had the lowest ORR of 9% (95% CI: 7%, 12%), while the combination of PD-1/PD-L1 inhibitors and chemotherapy had the highest ORR of 36% (95% CI: 24%, 51%). This study showed that PD-1/PD-L1 inhibitors alone have limited efficacy for ovarian cancer. The combination of PD-1/PD-L1 inhibitors and chemotherapy could be chosen as the recommended modality for further study.
Keywords: Immunotherapy; Ovarian cancer; PD-1/PD-L1 inhibitors.
© 2021. The Author(s).
Conflict of interest statement
The authors have stated that they have no conflicts of interest.
Figures




Similar articles
-
Safety and Efficacy of PD-1/PD-L1 inhibitors combined with radiotherapy in patients with non-small-cell lung cancer: a systematic review and meta-analysis.Cancer Med. 2021 Feb;10(4):1222-1239. doi: 10.1002/cam4.3718. Epub 2021 Jan 19. Cancer Med. 2021. PMID: 33465302 Free PMC article.
-
Anti-Tumor Efficacy of Anti-PD-1/PD-L1 Antibodies in Combination With Other Anticancer Drugs in Solid Tumors: A Systematic Review and Meta-Analysis.Cancer Control. 2022 Jan-Dec;29:10732748221140694. doi: 10.1177/10732748221140694. Cancer Control. 2022. PMID: 36748438 Free PMC article.
-
Clinical benefits of PD-1/PD-L1 inhibitors in advanced hepatocellular carcinoma: a systematic review and meta-analysis.Hepatol Int. 2020 Sep;14(5):765-775. doi: 10.1007/s12072-020-10064-8. Epub 2020 Jun 22. Hepatol Int. 2020. PMID: 32572818
-
Immune-checkpoint inhibitors plus chemotherapy versus chemotherapy as first-line treatment for patients with extensive-stage small cell lung cancer.J Immunother Cancer. 2020 Sep;8(2):e001300. doi: 10.1136/jitc-2020-001300. J Immunother Cancer. 2020. PMID: 32900864 Free PMC article.
-
PD-1 inhibitors for cutaneous squamous cell carcinoma: A meta-analysis.Australas J Dermatol. 2022 Feb;63(1):36-42. doi: 10.1111/ajd.13733. Epub 2021 Oct 26. Australas J Dermatol. 2022. PMID: 34699068 Review.
Cited by
-
Low plasma PD-L1 levels, early tumor onset and absence of peritoneal carcinomatosis improve prognosis of women with advanced high-grade serous ovarian cancer.BMC Cancer. 2023 May 13;23(1):437. doi: 10.1186/s12885-023-10911-5. BMC Cancer. 2023. PMID: 37179293 Free PMC article.
-
The role of PD-1/PD-L1 and application of immune-checkpoint inhibitors in human cancers.Front Immunol. 2022 Sep 13;13:964442. doi: 10.3389/fimmu.2022.964442. eCollection 2022. Front Immunol. 2022. PMID: 36177034 Free PMC article. Review.
-
Cervical cancer benefits from trabectedin combination with the β-blocker propranolol: in vitro and ex vivo evaluations in patient-derived organoids.Front Cell Dev Biol. 2023 Jun 13;11:1178316. doi: 10.3389/fcell.2023.1178316. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37384250 Free PMC article.
-
Immunotherapy for Platinum-Resistant Ovarian Cancer as a Glimmer of Hope.Cells. 2025 Jun 29;14(13):995. doi: 10.3390/cells14130995. Cells. 2025. PMID: 40643516 Free PMC article. Review.
-
Dysregulation of Peripheral Blood Mononuclear Cells and Immune-Related Proteins during the Early Post-Operative Immune Response in Ovarian Cancer Patients.Cancers (Basel). 2023 Dec 30;16(1):190. doi: 10.3390/cancers16010190. Cancers (Basel). 2023. PMID: 38201617 Free PMC article.
References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. - PubMed
-
- Matulonis UA, Shapira-Frommer R, Santin AD, Lisyanskaya AS, Pignata S, Vergote I, et al. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: results from the phase II KEYNOTE-100 study. Ann Oncol. 2019;30:1080–1087. doi: 10.1093/annonc/mdz135. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

