Extracellular protein components of amyloid plaques and their roles in Alzheimer's disease pathology
- PMID: 34454574
- PMCID: PMC8400902
- DOI: 10.1186/s13024-021-00465-0
Extracellular protein components of amyloid plaques and their roles in Alzheimer's disease pathology
Abstract
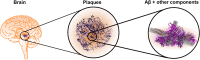
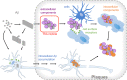
Alzheimer's disease (AD) is pathologically defined by the presence of fibrillar amyloid β (Aβ) peptide in extracellular senile plaques and tau filaments in intracellular neurofibrillary tangles. Extensive research has focused on understanding the assembly mechanisms and neurotoxic effects of Aβ during the last decades but still we only have a brief understanding of the disease associated biological processes. This review highlights the many other constituents that, beside Aβ, are accumulated in the plaques, with the focus on extracellular proteins. All living organisms rely on a delicate network of protein functionality. Deposition of significant amounts of certain proteins in insoluble inclusions will unquestionably lead to disturbances in the network, which may contribute to AD and copathology. This paper provide a comprehensive overview of extracellular proteins that have been shown to interact with Aβ and a discussion of their potential roles in AD pathology. Methods that can expand the knowledge about how the proteins are incorporated in plaques are described. Top-down methods to analyze post-mortem tissue and bottom-up approaches with the potential to provide molecular insights on the organization of plaque-like particles are compared. Finally, a network analysis of Aβ-interacting partners with enriched functional and structural key words is presented.
Keywords: Alzheimer’s disease; Amyloid corona; Amyloid-β; Protein interaction network; Senile plaque.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- World Alzheimer Report 2019. Attitudes to dementia. London: ADI. 2019.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

