A novel conjunctive microenvironment derived from human subcutaneous adipose tissue contributes to physiology of its superficial layer
- PMID: 34454629
- PMCID: PMC8399854
- DOI: 10.1186/s13287-021-02554-9
A novel conjunctive microenvironment derived from human subcutaneous adipose tissue contributes to physiology of its superficial layer
Abstract
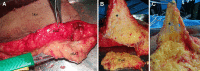
Background: In human subcutaneous adipose tissue, the superficial fascia distinguishes superficial and deep microenvironments showing extensions called retinacula cutis. The superficial subcutaneous adipose tissue has been described as hyperplastic and the deep subcutaneous adipose tissue as inflammatory. However, few studies have described stromal-vascular fraction (SVF) content and adipose-derived stromal/stem cells (ASCs) behavior derived from superficial and deep subcutaneous adipose tissue. In this study, we analyzed a third conjunctive microenvironment: the retinacula cutis superficialis derived from superficial subcutaneous adipose tissue.
Methods: The samples of abdominal human subcutaneous adipose tissue were obtained during plastic aesthetic surgery in France (Declaration DC-2008-162) and Brazil (Protocol 145/09).
Results: The SVF content was characterized in situ by immunofluorescence and ex vivo by flow cytometry revealing a high content of pre-adipocytes rather in superficial subcutaneous adipose tissue microenvironment. Adipogenic assays revealed higher percentage of lipid accumulation area in ASCs from superficial subcutaneous adipose tissue compared with retinacula cutis superficialis (p < 0.0001) and deep subcutaneous adipose tissue (p < 0.0001). The high adipogenic potential of superficial subcutaneous adipose tissue was corroborated by an up-regulation of adipocyte fatty acid-binding protein (FABP4) compared with retinacula cutis superficialis (p < 0.0001) and deep subcutaneous adipose tissue (p < 0.0001) and of C/EBPα (CCAAT/enhancer-binding protein alpha) compared with retinacula cutis superficialis (p < 0.0001) and deep subcutaneous adipose tissue (p < 0.0001) microenvironments. Curiously, ASCs from retinacula cutis superficialis showed a higher level of adiponectin receptor gene compared with superficial subcutaneous adipose tissue (p = 0.0409), widely known as an anti-inflammatory hormone. Non-induced ASCs from retinacula cutis superficialis showed higher secretion of human vascular endothelial growth factor (VEGF), compared with superficial (p = 0.0485) and deep (p = 0.0112) subcutaneous adipose tissue and with adipogenic-induced ASCs from superficial (p = 0.0175) and deep (p = 0.0328) subcutaneous adipose tissue. Furthermore, ASCs from retinacula cutis superficialis showed higher secretion of Chemokine (C-C motif) ligand 5 (CCL5) compared with non-induced (p = 0.0029) and induced (p = 0.0089) superficial subcutaneous adipose tissue.
Conclusions: This study highlights the contribution to ASCs from retinacula cutis superficialis in their angiogenic property previously described for the whole superficial subcutaneous adipose tissue besides supporting its adipogenic potential for superficial subcutaneous adipose tissue.
Keywords: Adipose stromal/stem cells; Deeper microenvironment; Human subcutaneous adipose tissue; Retinacula cutis microenvironment; Stromal vascular fraction; Superficial microenvironment.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Herlin C, Chica-Rosa A, Subsol G, Gilles B, Macri F, Beregi JP, et al. Three-dimensional study of the skin/subcutaneous complex using in vivo whole body 3T MRI: review of the literature and confirmation of a generic pattern of organization. Surg Radiol Anat. 2015;37(7):731–741. doi: 10.1007/s00276-014-1409-0. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

