1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study
- PMID: 34454673
- PMCID: PMC8389999
- DOI: 10.1016/S0140-6736(21)01755-4
1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study
Erratum in
-
Department of Error.Lancet. 2022 May 7;399(10337):1778. doi: 10.1016/S0140-6736(22)00795-4. Lancet. 2022. PMID: 35526552 Free PMC article. No abstract available.
Abstract
Background: The full range of long-term health consequences of COVID-19 in patients who are discharged from hospital is largely unclear. The aim of our study was to comprehensively compare consequences between 6 months and 12 months after symptom onset among hospital survivors with COVID-19.
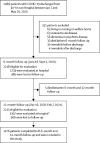
Methods: We undertook an ambidirectional cohort study of COVID-19 survivors who had been discharged from Jin Yin-tan Hospital (Wuhan, China) between Jan 7 and May 29, 2020. At 6-month and 12-month follow-up visit, survivors were interviewed with questionnaires on symptoms and health-related quality of life (HRQoL), and received a physical examination, a 6-min walking test, and laboratory tests. They were required to report their health-care use after discharge and work status at the 12-month visit. Survivors who had completed pulmonary function tests or had lung radiographic abnormality at 6 months were given the corresponding tests at 12 months. Non-COVID-19 participants (controls) matched for age, sex, and comorbidities were interviewed and completed questionnaires to assess prevalent symptoms and HRQoL. The primary outcomes were symptoms, modified British Medical Research Council (mMRC) score, HRQoL, and distance walked in 6 min (6MWD). Multivariable adjusted logistic regression models were used to evaluate the risk factors of 12-month outcomes.
Findings: 1276 COVID-19 survivors completed both visits. The median age of patients was 59·0 years (IQR 49·0-67·0) and 681 (53%) were men. The median follow-up time was 185·0 days (IQR 175·0-198·0) for the 6-month visit and 349·0 days (337·0-361·0) for the 12-month visit after symptom onset. The proportion of patients with at least one sequelae symptom decreased from 68% (831/1227) at 6 months to 49% (620/1272) at 12 months (p<0·0001). The proportion of patients with dyspnoea, characterised by mMRC score of 1 or more, slightly increased from 26% (313/1185) at 6-month visit to 30% (380/1271) at 12-month visit (p=0·014). Additionally, more patients had anxiety or depression at 12-month visit (26% [331/1271] at 12-month visit vs 23% [274/1187] at 6-month visit; p=0·015). No significant difference on 6MWD was observed between 6 months and 12 months. 88% (422/479) of patients who were employed before COVID-19 had returned to their original work at 12 months. Compared with men, women had an odds ratio of 1·43 (95% CI 1·04-1·96) for fatigue or muscle weakness, 2·00 (1·48-2·69) for anxiety or depression, and 2·97 (1·50-5·88) for diffusion impairment. Matched COVID-19 survivors at 12 months had more problems with mobility, pain or discomfort, and anxiety or depression, and had more prevalent symptoms than did controls.
Interpretation: Most COVID-19 survivors had a good physical and functional recovery during 1-year follow-up, and had returned to their original work and life. The health status in our cohort of COVID-19 survivors at 12 months was still lower than that in the control population.
Funding: Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences, the National Natural Science Foundation of China, the National Key Research and Development Program of China, Major Projects of National Science and Technology on New Drug Creation and Development of Pulmonary Tuberculosis, the China Evergrande Group, Jack Ma Foundation, Sino Biopharmaceutical, Ping An Insurance (Group), and New Sunshine Charity Foundation.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests We declare no competing interests.
Figures

Comment in
-
Understanding long COVID: a modern medical challenge.Lancet. 2021 Aug 28;398(10302):725. doi: 10.1016/S0140-6736(21)01900-0. Lancet. 2021. PMID: 34454656 Free PMC article. No abstract available.
-
Long-term effects on survivors with COVID-19.Lancet. 2021 Nov 20;398(10314):1871-1872. doi: 10.1016/S0140-6736(21)02283-2. Lancet. 2021. PMID: 34801100 Free PMC article. No abstract available.
-
Long-term effects on survivors with COVID-19.Lancet. 2021 Nov 20;398(10314):1872. doi: 10.1016/S0140-6736(21)02323-0. Lancet. 2021. PMID: 34801103 Free PMC article. No abstract available.
-
Post-COVID Dysautonomias: The Importance of Early Recognition and Implementation of Recovery Programs.Arq Bras Cardiol. 2023 Mar;120(3):e20230110. doi: 10.36660/abc.20230110. Arq Bras Cardiol. 2023. PMID: 37018794 Free PMC article. English, Portuguese. No abstract available.
References
-
- WHO WHO Coronavirus Disease (COVID-19) Dashboard. https://covid19.who.int/
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

