Astrocyte inflammatory signaling mediates α-synuclein aggregation and dopaminergic neuronal loss following viral encephalitis
- PMID: 34454938
- PMCID: PMC9535678
- DOI: 10.1016/j.expneurol.2021.113845
Astrocyte inflammatory signaling mediates α-synuclein aggregation and dopaminergic neuronal loss following viral encephalitis
Abstract
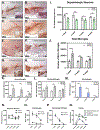
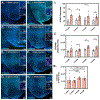
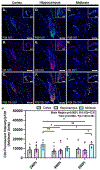
Viral infection of the central nervous system (CNS) can cause lasting neurological decline in surviving patients and can present with symptoms resembling Parkinson's disease (PD). The mechanisms underlying postencephalitic parkinsonism remain unclear but are thought to involve increased innate inflammatory signaling in glial cells, resulting in persistent neuroinflammation. We therefore studied the role of glial cells in regulating neuropathology in postencephalitic parkinsonism by studying the involvement of astrocytes in loss of dopaminergic neurons and aggregation of α-synuclein protein following infection with western equine encephalitis virus (WEEV). Infections were conducted in both wildtype mice and in transgenic mice lacking NFκB inflammatory signaling in astrocytes. For 2 months following WEEV infection, we analyzed glial activation, neuronal loss and protein aggregation across multiple brain regions, including the substantia nigra pars compacta (SNpc). These data revealed that WEEV induces loss of SNpc dopaminergic neurons, persistent activation of microglia and astrocytes that precipitates widespread aggregation of α-synuclein in the brain of C57BL/6 mice. Microgliosis and macrophage infiltration occurred prior to activation of astrocytes and was followed by opsonization of ⍺-synuclein protein aggregates in the cortex, hippocampus and midbrain by the complement protein, C3. Astrocyte-specific NFκB knockout mice had reduced gliosis, α-synuclein aggregate formation and neuronal loss. These data suggest that astrocytes play a critical role in initiating PD-like pathology following encephalitic infection with WEEV through innate immune inflammatory pathways that damage dopaminergic neurons, possibly by hindering clearance of ⍺-synuclein aggregates. Inhibiting glial inflammatory responses could therefore represent a potential therapy strategy for viral parkinsonism.
Keywords: Alpha-synuclein; Alphaviruses; Glia; Neurodegeneration; Neuroinflammation; Parkinson’s Disease; Viral encephalitis; Western equine encephalitis virus.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Competing Interest
The authors declare no competing interest
Competing interests
The authors declare that there exist no competing interests or conflicts, disclosed or otherwise. All research and data will be made freely accessible, per the guidelines of the National Institutes of Health.
Collin M. Bantle
Savannah M. Rocha
C. Tenley French
Aaron T. Phillips
Kevin Tran
Kenneth E. Olson
Todd A. Bass
Tawfik Aboellail
Richard J. Smeyne
Ronald B. Tjalkens
Figures





References
-
- Anderson JP, et al. 2006. Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. J Biol Chem. 281, 29739–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

