Analysis of antibody responses after COVID-19 vaccination in liver transplant recipients and those with chronic liver diseases
- PMID: 34454993
- PMCID: PMC8387568
- DOI: 10.1016/j.jhep.2021.08.008
Analysis of antibody responses after COVID-19 vaccination in liver transplant recipients and those with chronic liver diseases
Abstract
Background & aims: Liver transplant (LT) recipients or other immunocompromised patients were not included in the registration trials studying the efficacy of vaccines against SARS-CoV-2. Although the clinical efficacy of COVID-19 vaccines in immunocompromised patients is unknown, many societies have recommended vaccination of this highly vulnerable patient population.
Methods: In this prospective study, we determined antibody responses to spike protein, 4 weeks after the 2nd dose of mRNA vaccines or after the single dose of Johnson & Johnson vaccine, in LT recipients and those with chronic liver disease (CLD) with and without cirrhosis.
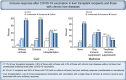
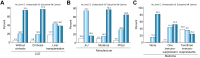
Results: Of the 233 patients enrolled so far, 62 were LT recipients, 79 had cirrhosis (10 decompensated) and 92 had CLD without cirrhosis. Antibody titers were defined as undetectable (<0.40 U/ml), suboptimal (0.40-250 U/ml) and adequate (>250 U/ml). Of the 62 patients who had LT, antibody levels were undetectable in 11 patients and suboptimal (median titer 17.6, range 0.47-212 U/ml) in 27 patients. Among 79 patients with cirrhosis, 3 had undetectable antibody levels and 15 had suboptimal (median titer 41.3, range 0.49-221 U/L) antibody responses. Of the 92 patients without cirrhosis, 4 had undetectable antibody levels and 19 had suboptimal (median titer 95.5, range 4.9-234 U/L) antibody responses. Liver transplantation, use of 2 or more immunosuppression medications and vaccination with a single dose of the Johnson & Johnson vaccine were associated with poor immune response on multivariable analysis. No patient had any serious adverse events.
Conclusions: Poor antibody responses after SARS-CoV-2 vaccination were seen in 61% of LT recipients and 24% of those with CLD.
Lay summary: The clinical efficacy of COVID-19 vaccines in immunocompromised patients is unknown. We performed a prospective study to evaluate immune responses to COVID-19 vaccines (Moderna, Pfizer or Johnson & Johnson) in 62 liver transplant recipients, 79 patients with cirrhosis and 92 with chronic liver diseases without cirrhosis. We found that 17.8% of liver transplant recipients, 3.8% of those with cirrhosis and 4.3% of those with chronic liver diseases without cirrhosis had undetectable antibody levels. In total, 61.3% of liver transplant recipients and 24% of those with chronic liver diseases (with or without cirrhosis) had poor antibody responses (undetectable or suboptimal). Liver transplantation, use of immunosuppressive medications and vaccination with a single dose of Johnson & Johnson vaccine were associated with poor antibody responses when adjusted for other factors.
Keywords: SARS-CoV-2; cirrhosis; immunocompromised; liver transplant; mRNA vaccine.
Copyright © 2021 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare no conflicts of interest that pertain to this work. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures
References
-
- Belli L.S., Fondevila C., Cortesi P.A., Conti S., Karam V., Adam R., et al. for ELITA-ELTR COVID-19 Registry Protective role of tacrolimus, deleterious role of age and comorbidities in liver transplant recipients with Covid-19: results from the ELITA/ELTR Multi-center European Study. Gastroenterology. 2021 Mar;160(4):1151–1163. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

