Single-Fraction vs Multifraction Stereotactic Ablative Body Radiotherapy for Pulmonary Oligometastases (SAFRON II): The Trans Tasman Radiation Oncology Group 13.01 Phase 2 Randomized Clinical Trial
- PMID: 34455431
- PMCID: PMC8404145
- DOI: 10.1001/jamaoncol.2021.2939
Single-Fraction vs Multifraction Stereotactic Ablative Body Radiotherapy for Pulmonary Oligometastases (SAFRON II): The Trans Tasman Radiation Oncology Group 13.01 Phase 2 Randomized Clinical Trial
Abstract
Importance: Evidence is lacking from randomized clinical trials to guide the optimal approach for stereotactic ablative body radiotherapy (SABR) in patients with pulmonary oligometastases.
Objective: To assess whether single-fraction or multifraction SABR is more effective for the treatment of patients with pulmonary oligometastases.
Design, setting, and participants: This multicenter, unblinded, phase 2 randomized clinical trial of 90 patients across 13 centers in Australia and New Zealand enrolled patients with 1 to 3 lung oligometastases less than or equal to 5 cm from any nonhematologic malignant tumors located away from the central airways, Eastern Cooperative Oncology Group performance status 0 or 1, and all primary and extrathoracic disease controlled with local therapy. Enrollment was from January 1, 2015, to December 31, 2018, with a minimum patient follow-up of 2 years.
Interventions: Single fraction of 28 Gy (single-fraction arm) or 4 fractions of 12 Gy (multifraction arm) to each oligometastasis.
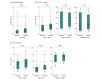
Main outcomes and measures: The main outcome was grade 3 or higher treatment-related adverse events (AEs) occurring within 1 year of SABR. Secondary outcomes were freedom from local failure, overall survival, disease-free survival, and patient-reported outcomes (MD Anderson Symptom Inventory-Lung Cancer and EuroQol 5-dimension visual analog scale).
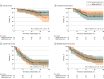
Results: Ninety participants were randomized, of whom 87 were treated for 133 pulmonary oligometastases. The mean (SD) age was 66.6 [11.6] years; 58 (64%) were male. Median follow-up was 36.5 months (interquartile range, 24.8-43.9 months). The numbers of grade 3 or higher AEs related to treatment at 1 year were 2 (5%; 80% CI, 1%-13%) in the single-fraction arm and 1 (3%; 80% CI, 0%-10%) in the multifraction arm, with no significant difference observed between arms. One grade 5 AE occurred in the multifraction arm. No significant differences were found between the multifraction arm and single-fraction arm for freedom from local failure (hazard ratio [HR], 0.5; 95% CI, 0.2-1.3; P = .13), overall survival (HR, 1.5; 95% CI, 0.6-3.7; P = .44), or disease-free survival (HR, 1.0; 95% CI, 0.6-1.6; P > .99). There were no significant differences observed in patient-reported outcomes.
Conclusions and relevance: In this randomized clinical trial, neither arm demonstrated evidence of superior safety, efficacy, or symptom burden; however, single-fraction SABR is more efficient to deliver. Therefore, single-fraction SABR, as assessed by the most acceptable outcome profile from all end points, could be chosen to escalate to future studies.
Trial registration: ClinicalTrials.gov Identifier: NCT01965223.
Conflict of interest statement
Figures


Comment in
-
Managing Pulmonary Oligometastatic Disease With Stereotactic Body Radiation Therapy-Moving the Field Forward 1 Organ at a Time.JAMA Oncol. 2021 Oct 1;7(10):1485-1486. doi: 10.1001/jamaoncol.2021.2926. JAMA Oncol. 2021. PMID: 34455429 No abstract available.
References
-
- Gomez DR, Tang C, Zhang J, et al. . Local consolidative therapy vs. maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer: long-term results of a multi-institutional, phase ii, randomized study. J Clin Oncol. 2019;37(18):1558-1565. doi:10.1200/JCO.19.00201 - DOI - PMC - PubMed
-
- Videtic GM, Paulus R, Singh AK, et al. . Long-term follow-up on NRG Oncology RTOG 0915 (NCCTG N0927): a randomized phase 2 study comparing 2 stereotactic body radiation therapy schedules for medically inoperable patients with stage I peripheral non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2019;103(5):1077-1084. doi:10.1016/j.ijrobp.2018.11.051 - DOI - PMC - PubMed

