Mapping Migraine-Specific Quality of Life to Health State Utilities in Patients Receiving Rimegepant
- PMID: 34455556
- PMCID: PMC8478726
- DOI: 10.1007/s12325-021-01897-2
Mapping Migraine-Specific Quality of Life to Health State Utilities in Patients Receiving Rimegepant
Abstract
Introduction: Migraine is a debilitating neurological condition, affecting up to 15% of Americans. Recent estimates from a long-term safety study of rimegepant showed evidence of decreased monthly migraine days (MMD) in people with episodic migraine treated with rimegepant 75 mg. The objective of this study was to characterize migraine-specific quality of life version 2.1 (MSQv2) scores and corresponding mapped EuroQol-5 Dimensions-3 Level (EQ-5D-3L) utility values.
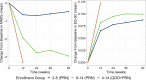
Methods: Study participants were randomized into two treatment regimens: individuals with 2-14 MMD received rimegepant 75 mg as needed (PRN), and those with 4-14 MMD at baseline who received rimegepant on a fixed every-other-day schedule plus an as needed dose on days they did not treat (QOD + PRN). MSQv2 was mapped to EQ-5D-3L utilities using a validated algorithm. Outcomes were assessed for the PRN arm at baseline weeks 12, 24, 36, and 52 and for the QOD + PRN arm at baseline and week 12.
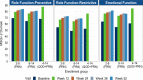
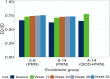
Results: At baseline, MSQv2 data were available for 1,800 patients: 1,033 with 2-8 MMD in the PRN group, 481 with 9-14 MMD in the PRN group, and 286 with 4-14 MMD in the QOD + PRN group. For all MSQv2 domains as well as mapped utility values, outcomes improved over each study visit. At baseline, EQ-5D-3L utilities were 0.66, 0.63, and 0.65 for the 2-8 MMD PRN, 9-14 MMD PRN, and 4-14 MMD QOD + PRN groups, respectively. At end-of-study, utilities had increased by + 0.09, + 0.10, and + 0.12 for the three groups, respectively (p < 0.001 for all comparisons with baseline). Similar trends in improvement were observed across MSQv2 subdomains; all differences were statistically significant.
Conclusions: Rimegepant 75 mg, which has been shown to be associated with reduced MMD, is associated with improvement in MSQv2 domains over time, leading to estimated improvement in EQ-5D-3L utilities. While this improvement was observed in all patient-groups, it was most pronounced in those with higher MMD and those taking rimegepant QOD + PRN.
Trial registration: Clinical Trials NCT03266588.
Keywords: EQ-5D; Mapping; Migraine; Migraine-specific quality of life (MSQv2); Patient-reported outcome; Preference-based instrument; Utility.
© 2021. The Author(s).
Figures
Similar articles
-
Health State Utility Mapping of Rimegepant for the Preventive Treatment of Migraine: Double-Blind Treatment Phase and Open Label Extension (BHV3000-305).Adv Ther. 2023 Feb;40(2):585-600. doi: 10.1007/s12325-022-02369-x. Epub 2022 Nov 22. Adv Ther. 2023. PMID: 36417057 Free PMC article. Clinical Trial.
-
Monthly migraine days, tablet utilization, and quality of life associated with Rimegepant - post hoc results from an open label safety study (BHV3000-201).J Headache Pain. 2022 Jan 17;23(1):10. doi: 10.1186/s10194-021-01378-5. J Headache Pain. 2022. PMID: 35038983 Free PMC article. Clinical Trial.
-
Matching-adjusted indirect comparisons of oral rimegepant versus placebo, erenumab, and galcanezumab examining monthly migraine days and health-related quality of life in the treatment of migraine.Headache. 2021 Jun;61(6):906-915. doi: 10.1111/head.14128. Epub 2021 May 22. Headache. 2021. PMID: 34021585 Free PMC article.
-
Comparative efficacy, quality of life, safety, and tolerability of atogepant and rimegepant in migraine prevention: A matching-adjusted indirect comparison analysis.Cephalalgia. 2024 Feb;44(2):3331024241235156. doi: 10.1177/03331024241235156. Cephalalgia. 2024. PMID: 38410850 Review.
-
Small molecule CGRP receptor antagonists for the preventive treatment of migraine: A review.Eur J Pharmacol. 2022 May 5;922:174902. doi: 10.1016/j.ejphar.2022.174902. Epub 2022 Mar 28. Eur J Pharmacol. 2022. PMID: 35358493 Review.
Cited by
-
Rimegepant: A Review in the Acute Treatment and Preventive Treatment of Migraine.CNS Drugs. 2023 Mar;37(3):255-265. doi: 10.1007/s40263-023-00988-8. Epub 2023 Feb 4. CNS Drugs. 2023. PMID: 36739335 Free PMC article. Review.
-
Pharmacokinetics of Atogepant in Healthy Lactating Female Participants: Results from a Phase 1 Lactation Study.Neurol Ther. 2025 Aug;14(4):1461-1473. doi: 10.1007/s40120-025-00772-4. Epub 2025 Jun 2. Neurol Ther. 2025. PMID: 40455369 Free PMC article.
-
Clinical efficacy and safety of rimegepant in the treatment of migraine: a meta-analysis of randomized controlled trials.Front Neurol. 2023 Jun 20;14:1205778. doi: 10.3389/fneur.2023.1205778. eCollection 2023. Front Neurol. 2023. PMID: 37409024 Free PMC article.
-
Profile of Sensory Integration Disorders in Migraine Patients-New Perspectives of Therapy.J Clin Med. 2024 Jul 4;13(13):3928. doi: 10.3390/jcm13133928. J Clin Med. 2024. PMID: 38999493 Free PMC article.
References
-
- American Headache Society AHS consensus statement: the american headache society position statement on integrating new migraine treatments into clinical practice. Headache. 2019;59:1–18. - PubMed
-
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38(1):1–211. - PubMed
-
- Mayans L, Walling A. Acute Migraine headache: treatment strategies. Am Fam Physician. 2018;97(4):243–251. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

