Bioinspired artificial platelets: past, present and future
- PMID: 34455908
- PMCID: PMC8795470
- DOI: 10.1080/09537104.2021.1967916
Bioinspired artificial platelets: past, present and future
Abstract
Platelets are anucleate blood cells produced from megakaryocytes predominantly in the bone marrow and released into blood circulation at a healthy count of 150,000-400,00 per μL and circulation lifespan of 7-9 days. Platelets are the first responders at the site of vascular injury and bleeding, and participate in clot formation via injury site-specific primary mechanisms of adhesion, activation and aggregation to form a platelet plug, as well as secondary mechanisms of augmenting coagulation via thrombin amplification and fibrin generation. Platelets also secrete various granule contents that enhance these mechanisms for clot growth and stability. The resultant clot seals the injury site to stanch bleeding, a process termed as hemostasis. Due to this critical role, a reduction in platelet count or dysregulation in platelet function is associated with bleeding risks and hemorrhagic complications. These scenarios are often treated by prophylactic or emergency transfusion of platelets. However, platelet transfusions face significant challenges due to limited donor availability, difficult portability and storage, high bacterial contamination risks, and very short shelf life (~5-7 days). These are currently being addressed by a robust volume of research involving reduced temperature storage and pathogen reduction processes on donor platelets to improve shelf-life and reduce contamination, as well as bioreactor-based approaches to generate donor-independent platelets from stem cells in vitro. In parallel, a complementary research field has emerged that involves the design of artificial platelets utilizing biosynthetic particle constructs that functionally emulate various hemostatic mechanisms of platelets. Here, we provide a comprehensive review of the history and the current state-of-the-art artificial platelet approaches, along with discussing the translational opportunities and challenges.
Keywords: Artificial Platelets; Bioinspired; Haemostasis; Platelets; Transfusion.
Figures

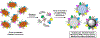



Similar articles
-
Platelet-inspired nanomedicine in hemostasis thrombosis and thromboinflammation.J Thromb Haemost. 2022 Jul;20(7):1535-1549. doi: 10.1111/jth.15734. Epub 2022 Apr 26. J Thromb Haemost. 2022. PMID: 35435322 Free PMC article. Review.
-
In vitro characterization of SynthoPlate™ (synthetic platelet) technology and its in vivo evaluation in severely thrombocytopenic mice.J Thromb Haemost. 2017 Feb;15(2):375-387. doi: 10.1111/jth.13579. J Thromb Haemost. 2017. PMID: 27925685 Free PMC article.
-
Bioinspired artificial platelets for transfusion applications in traumatic hemorrhage.Transfusion. 2020 Feb;60(2):229-231. doi: 10.1111/trf.15543. Epub 2019 Oct 18. Transfusion. 2020. PMID: 31625169 Free PMC article. Review.
-
Current challenges in platelet transfusion.Platelets. 2022 Jan 2;33(1):5-13. doi: 10.1080/09537104.2021.1961711. Epub 2021 Aug 16. Platelets. 2022. PMID: 34392769 Review.
-
[Single-donor (apheresis) platelets and pooled whole-blood-derived platelets--significance and assessment of both blood products].Clin Lab. 2014;60(4):S1-39. doi: 10.7754/clin.lab.2014.140210. Clin Lab. 2014. PMID: 24779310 Review. German.
Cited by
-
Nomogram and Risk Calculator for Postoperative Tracheostomy after Heart Valve Surgery.J Cardiovasc Dev Dis. 2023 Feb 8;10(2):73. doi: 10.3390/jcdd10020073. J Cardiovasc Dev Dis. 2023. PMID: 36826569 Free PMC article.
-
[Role and prospect of regenerative medicine in early treatment of combat trauma].Zhonghua Shao Shang Yu Chuang Mian Xiu Fu Za Zhi. 2023 May 20;39(5):411-416. doi: 10.3760/cma.j.cn501225-20220419-00147. Zhonghua Shao Shang Yu Chuang Mian Xiu Fu Za Zhi. 2023. PMID: 37805749 Free PMC article. Chinese.
-
Artificial blood-hope and the challenges to combat tumor hypoxia for anti-cancer therapy.Med Biol Eng Comput. 2025 Apr;63(4):933-957. doi: 10.1007/s11517-024-03233-6. Epub 2024 Nov 30. Med Biol Eng Comput. 2025. PMID: 39614063 Review.
-
Efficacy of platelet-inspired hemostatic nanoparticles on bleeding in von Willebrand disease murine models.Blood. 2023 Jun 8;141(23):2891-2900. doi: 10.1182/blood.2022018956. Blood. 2023. PMID: 36928925 Free PMC article.
-
ENGINEERED INTRAVENOUS THERAPIES FOR TRAUMA.Curr Opin Biomed Eng. 2023 Sep;27:100456. doi: 10.1016/j.cobme.2023.100456. Epub 2023 Mar 23. Curr Opin Biomed Eng. 2023. PMID: 37456984 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
