Acoustic Change Complex and Visually Reinforced Infant Speech Discrimination Measures of Vowel Contrast Detection
- PMID: 34456301
- PMCID: PMC8873241
- DOI: 10.1097/AUD.0000000000001116
Acoustic Change Complex and Visually Reinforced Infant Speech Discrimination Measures of Vowel Contrast Detection
Abstract
Objectives: To measure the effect of stimulus rate and vowel change direction on the acoustic change complex (ACC) latencies and amplitudes and compare ACC metrics to behavioral measures of vowel contrast detection for infants tested under the age of 1 year. We tested the hypothesis that the direction of spectral energy shift from a vowel change would result in differences in the ACC, owing to the sensitivity of cortical neurons to the direction of frequency change. We evaluated the effect of the stimulus rate (1/s versus 2/s) on the infants' ACC. We evaluated the ACC amplitude ratio's sensitivity (proportion of ACCs present for each change trial) and compared it to perceptual responses obtained using a visually reinforced infant speech discrimination paradigm (VRISD). This report provides normative data from infants for the ACC toward the ultimate goal of developing a clinically useful index of neural capacity for vowel discrimination.
Design: Twenty-nine infants, nine females, 4.0 to 11.8 months of age, participated. All participants were born at full term and passed their newborn hearing screens. None had risk factors for hearing or neurologic impairment. Cortical auditory evoked potentials were obtained in response to synthesized vowel tokens /a/, /i/, /o/, and /u/ presented at a rate of 1- or 2/s in an oddball stimulus paradigm with a 25% probability of the deviant stimulus. All combinations of vowel tokens were tested at the two rates. The ACC was obtained in response to the deviant stimulus. The infants were also tested for vowel contrast detection using a VRISD paradigm with the same combinations of vowel tokens used for the ACC. The mean age at the time of the ACC test was 5.4 months, while the mean age at the behavioral test was 6.8 months.
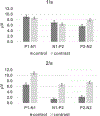
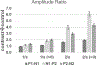
Results: Variations in ACC amplitude and latency occurred as a function of the initial vowel token and the contrast token. However, the hypothesis that the direction of vowel (spectral) change would result in significantly larger change responses for high-to-low spectral changes was not supported. The contrasts with /a/ as the leading vowel of the contrast pair resulted in the largest ACC amplitudes than other conditions. Significant differences in the ACC presence and amplitude were observed as a function of rate, with 2/s resulting in ACCs with the largest amplitude ratios. Latency effects of vowel contrast and rate were present, but not systematic. The ACC amplitude ratio's sensitivity for detecting a vowel contrast was greater for the 2/s rate than the 1/s rate. For an amplitude ratio criterion of ≥1.5, the sensitivity was 93% for ACC component P2-N2 at 2/s, whereas at 1/s sensitivity was 70%. VRISD tests of vowel-contrast detection had a 71% hit and a 21% false-positive rate. Many infants who could not reach performance criteria for VRISD had ACC amplitude ratios of ≥2.0.
Conclusions: The ACC for vowel contrasts presented at a rate of 2/s is a robust index of vowel-contrast detection when obtained in typically developing infants under the age of 1 year. The ACC is present in over 90% of infants tested at this rate when an amplitude ratio criterion of ≥1.5 is used to define a response. The amplitude ratio appears to be a sensitive metric for the difference between a control and contrast condition. The ACC can be obtained in infants who do not yet exhibit valid behavioral responses for vowel change contrasts and may be useful for estimating neural capacity for discriminating these sounds.
Copyright © 2021 Wolters Kluwer Health, Inc. All rights reserved.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures
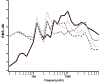


 CAEP onset response to standard vowel token
CAEP onset response to standard vowel token  ACC response in control condition
ACC response in control condition  ACC response in contrast conditions, /a/, /o/ /i/ or /u/ ACC waveforms shown in alphabetical order for each condition: for /i/, the contrasts shown are /a/, /o/ and /u/. For /o/, the contrast are /a/, /i/ and /u/. For /u/, the contrasts are /a/, /i/, /o/. Markers have been provided for response components in some waveforms as a guide for identifying the components in the subsequent waveforms. ACCs are identified as differences in amplitude between the control (thin gray trace) and contrast (thin black trace) conditions. In the 1/s example, ACCs for the /u/ condition are questionable for the /i/ and /o/ (bottom two) traces. As described in the text, CAEP onset responses to the standard vowel token are frequetly absent at the 2/s rate and this is the case in the examples found for /o/ and /u/. Examples of waveforms for /a/ standards, controls and /i/, /o/ and /u/ contrasts are found in Cone (2015).
ACC response in contrast conditions, /a/, /o/ /i/ or /u/ ACC waveforms shown in alphabetical order for each condition: for /i/, the contrasts shown are /a/, /o/ and /u/. For /o/, the contrast are /a/, /i/ and /u/. For /u/, the contrasts are /a/, /i/, /o/. Markers have been provided for response components in some waveforms as a guide for identifying the components in the subsequent waveforms. ACCs are identified as differences in amplitude between the control (thin gray trace) and contrast (thin black trace) conditions. In the 1/s example, ACCs for the /u/ condition are questionable for the /i/ and /o/ (bottom two) traces. As described in the text, CAEP onset responses to the standard vowel token are frequetly absent at the 2/s rate and this is the case in the examples found for /o/ and /u/. Examples of waveforms for /a/ standards, controls and /i/, /o/ and /u/ contrasts are found in Cone (2015).
References
-
- Casey KA, Small SA (2014) Comparisons of auditory steady-state response and behavioral air conduction and bone conduction thresholds for infants and adults with normal hearing. Ear and Hearing 35(4): 423–439. - PubMed
-
- Cheek D, Cone B (2020) Evidence of vowel discrimination provided by the acoustic change complex. Ear and Hearing 41(4) 855–867. - PubMed
-
- Cheour M, Lepannen PH, Kraus N (2000) Mismatch negativity (MMN) as a tool for investigating auditory discrimination and sensory memory in infants and children. Clinical Neurophysiology 111 (1), 4–16. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

