The Amyloid-β Pathway in Alzheimer's Disease
- PMID: 34456336
- PMCID: PMC8758495
- DOI: 10.1038/s41380-021-01249-0
The Amyloid-β Pathway in Alzheimer's Disease
Abstract
Breakthroughs in molecular medicine have positioned the amyloid-β (Aβ) pathway at the center of Alzheimer's disease (AD) pathophysiology. While the detailed molecular mechanisms of the pathway and the spatial-temporal dynamics leading to synaptic failure, neurodegeneration, and clinical onset are still under intense investigation, the established biochemical alterations of the Aβ cycle remain the core biological hallmark of AD and are promising targets for the development of disease-modifying therapies. Here, we systematically review and update the vast state-of-the-art literature of Aβ science with evidence from basic research studies to human genetic and multi-modal biomarker investigations, which supports a crucial role of Aβ pathway dyshomeostasis in AD pathophysiological dynamics. We discuss the evidence highlighting a differentiated interaction of distinct Aβ species with other AD-related biological mechanisms, such as tau-mediated, neuroimmune and inflammatory changes, as well as a neurochemical imbalance. Through the lens of the latest development of multimodal in vivo biomarkers of AD, this cross-disciplinary review examines the compelling hypothesis- and data-driven rationale for Aβ-targeting therapeutic strategies in development for the early treatment of AD.
© 2021. The Author(s).
Conflict of interest statement
HH is an employee of Eisai Inc. HH serves as Senior Associate Editor for the Journal Alzheimer’s & Dementia and does not receive any fees or honoraria since May 2019; before May 2019 he had received lecture fees from Servier, Biogen and Roche, research grants from Pfizer, Avid, and MSD Avenir (paid to the institution), travel funding from Eisai, Functional Neuromodulation, Axovant, Eli Lilly and company, Takeda and Zinfandel, GE-Healthcare and Oryzon Genomics, consultancy fees from Qynapse, Jung Diagnostics, Cytox Ltd., Axovant, Anavex, Takeda and Zinfandel, GE Healthcare, Oryzon Genomics, and Functional Neuromodulation, and participated in scientific advisory boards of Functional Neuromodulation, Axovant, Eisai, Eli Lilly and company, Cytox Ltd., GE Healthcare, Takeda and Zinfandel, Oryzon Genomics and Roche Diagnostics. He is co-inventor in the following patents as a scientific expert and has received no royalties: • In Vitro Multiparameter Determination Method for The Diagnosis and Early Diagnosis of Neurodegenerative Disorders Patent Number: 8916388. • In Vitro Procedure for Diagnosis and Early Diagnosis of Neurodegenerative Diseases Patent Number: 8298784. • Neurodegenerative Markers for Psychiatric Conditions Publication Number: 20120196300. • In Vitro Multiparameter Determination Method for The Diagnosis and Early Diagnosis of Neurodegenerative Disorders Publication Number: 20100062463. • In Vitro Method for The Diagnosis and Early Diagnosis of Neurodegenerative Disorders Publication Number: 20100035286. • In Vitro Procedure for Diagnosis and Early Diagnosis of Neurodegenerative Diseases Publication Number: 20090263822. • In Vitro Method for The Diagnosis of Neurodegenerative Diseases Patent Number: 7547553. • CSF Diagnostic in Vitro Method for Diagnosis of Dementias and Neuroinflammatory Diseases Publication Number: 20080206797. • In Vitro Method for The Diagnosis of Neurodegenerative Diseases Publication Number: 20080199966. • Neurodegenerative Markers for Psychiatric Conditions Publication Number: 20080131921. • Method for diagnosis of dementias and neuroinflammatory diseases based on an increased level of procalcitonin in cerebrospinal fluid: Publication number: United States Patent 10921330.
Figures
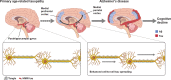
Comment in
-
The amyloid-β pathway in Alzheimer's disease: a plain language summary.Neurodegener Dis Manag. 2023 Jun;13(3):141-149. doi: 10.2217/nmt-2022-0037. Epub 2023 Mar 30. Neurodegener Dis Manag. 2023. PMID: 36994753 Free PMC article.
References
-
- Prince MJ, Wimo A, Guerchet MM, Ali GC, Wu Y-T, Prina M. World Alzheimer Report 2015 - The Global Impact of Dementia: An analysis of prevalence, incidence, cost and trends. London: Alzheimer’s Disease International, 2015. 84 p.
-
- 2020 Alzheimer’s disease facts and figures. Alzheimer’s & Dement. 2020;16:391–460. - PubMed
-
- Prince M, Knapp M, Guerchet M, McCrone P, Prina M, Comas-Herrera A et al. Dementia UK: Second Edition—Overview. Alzheimer’s Soc. 2014.
-
- Canevelli M, Lacorte E, Cova I, Zaccaria V, Valletta M, Raganato R, et al. Estimating dementia cases amongst migrants living in Europe. Eur J Neurol. 2019;26:1191–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

