Drugs that Might Be Possibly Used for Treatment of COVID-19 Patients
- PMID: 34456540
- PMCID: PMC8380022
- DOI: 10.1134/S1068162021040130
Drugs that Might Be Possibly Used for Treatment of COVID-19 Patients
Abstract
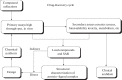
The drug development process for Coronavirus disease (COVID-19) is the research process to create a preventive vaccine or therapeutic prescription drug to relieve the severity of 2019-2020 (COVID-19). In different stages of preclinical or clinical research, several hundred special scientific research centers, research organizations, and health agencies have developed and tried enormous numbers of vaccine candidates and new drugs for COVID-19 disease. In order to identify new therapies against COVID-19, several clinical trials have been in progress worldwide.
Keywords: Coronavirus disease; Favipiravir; Losartan; drug development process; trial of treatments.
© Pleiades Publishing, Ltd. 2021, ISSN 1068-1620, Russian Journal of Bioorganic Chemistry, 2021, Vol. 47, No. 4, pp. 789–804. © Pleiades Publishing, Ltd., 2021.
Conflict of interest statement
Conflict of InterestsThe authors report no conflicts of interest.
Figures





References
-
- Goniewicz K., Khorram-Manesh A., Hertelendy A. J., Goniewicz M., Naylor K., Burkle M. F. Sustainability. 2020;12:3838. doi: 10.3390/su12093838. - DOI
LinkOut - more resources
Full Text Sources
