Adenylate Cyclase 1 Links Calcium Signaling to CFTR-Dependent Cytosolic Chloride Elevations in Chick Amacrine Cells
- PMID: 34456687
- PMCID: PMC8385318
- DOI: 10.3389/fncel.2021.726605
Adenylate Cyclase 1 Links Calcium Signaling to CFTR-Dependent Cytosolic Chloride Elevations in Chick Amacrine Cells
Abstract
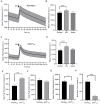
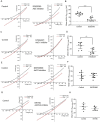
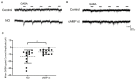
The strength and sign of synapses involving ionotropic GABA and glycine receptors are dependent upon the Cl- gradient. We have shown that nitric oxide (NO) elicits the release of Cl- from internal acidic stores in retinal amacrine cells (ACs); temporarily altering the Cl- gradient and the strength or even sign of incoming GABAergic or glycinergic synapses. The underlying mechanism for this effect of NO requires the cystic fibrosis transmembrane regulator (CFTR) but the link between NO and CFTR activation has not been determined. Here, we test the hypothesis that NO-dependent Ca2+ elevations activate the Ca2+-dependent adenylate cyclase 1 (AdC1) leading to activation of protein kinase A (PKA) whose activity is known to open the CFTR channel. Using the reversal potential of GABA-gated currents to monitor cytosolic Cl-, we established the requirement for Ca2+ elevations. Inhibitors of AdC1 suppressed the NO-dependent increases in cytosolic Cl- whereas inhibitors of other AdC subtypes were ineffective suggesting that AdC1 is involved. Inhibition of PKA also suppressed the action of NO. To address the sufficiency of this pathway in linking NO to elevations in cytosolic Cl-, GABA-gated currents were measured under internal and external zero Cl- conditions to isolate the internal Cl- store. Activators of the cAMP pathway were less effective than NO in producing GABA-gated currents. However, coupling the cAMP pathway activators with the release of Ca2+ from stores produced GABA-gated currents indistinguishable from those stimulated with NO. Together, these results demonstrate that cytosolic Ca2+ links NO to the activation of CFTR and the elevation of cytosolic Cl-.
Keywords: CFTR; Ca2+; GABAergic neuron; adenylate cyclase 1; amacrine cell; cAMP; nitric oxide.
Copyright © 2021 Zhong and Gleason.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Reduced Expression of TMEM16A Impairs Nitric Oxide-Dependent Cl- Transport in Retinal Amacrine Cells.Front Cell Neurosci. 2022 Jul 27;16:937060. doi: 10.3389/fncel.2022.937060. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35966201 Free PMC article.
-
A role for the cystic fibrosis transmembrane conductance regulator in the nitric oxide-dependent release of Cl- from acidic organelles in amacrine cells.J Neurophysiol. 2017 Nov 1;118(5):2842-2852. doi: 10.1152/jn.00511.2017. Epub 2017 Aug 23. J Neurophysiol. 2017. PMID: 28835528 Free PMC article.
-
Inhibition of endocytosis suppresses the nitric oxide-dependent release of Cl- in retinal amacrine cells.PLoS One. 2018 Jul 25;13(7):e0201184. doi: 10.1371/journal.pone.0201184. eCollection 2018. PLoS One. 2018. PMID: 30044876 Free PMC article.
-
CFTR: a hub for kinases and crosstalk of cAMP and Ca2+.FEBS J. 2013 Sep;280(18):4417-29. doi: 10.1111/febs.12457. Epub 2013 Aug 27. FEBS J. 2013. PMID: 23895508 Review.
-
Role of the actin cytoskeleton in the regulation of the cystic fibrosis transmembrane conductance regulator.Exp Physiol. 1996 May;81(3):505-14. doi: 10.1113/expphysiol.1996.sp003953. Exp Physiol. 1996. PMID: 8737083 Review.
Cited by
-
Second-Generation Antipsychotics Induce Metabolic Disruption in Adipose Tissue-Derived Mesenchymal Stem Cells Through an aPKC-Dependent Pathway.Cells. 2024 Dec 17;13(24):2084. doi: 10.3390/cells13242084. Cells. 2024. PMID: 39768174 Free PMC article.
-
Reduced Expression of TMEM16A Impairs Nitric Oxide-Dependent Cl- Transport in Retinal Amacrine Cells.Front Cell Neurosci. 2022 Jul 27;16:937060. doi: 10.3389/fncel.2022.937060. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35966201 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

